Preparation methods for fluorine-containing thienopyrrolodione monomer and copolymer thereof
A technology of diketopyrrole and copolymer, which is applied in the field of preparation of fluorine-containing diketopyrrole monomer and its copolymer, can solve the problems of poor thermal stability and insufficient light absorption range, and achieve improved thermal stability, Effects of improving life and reducing energy gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
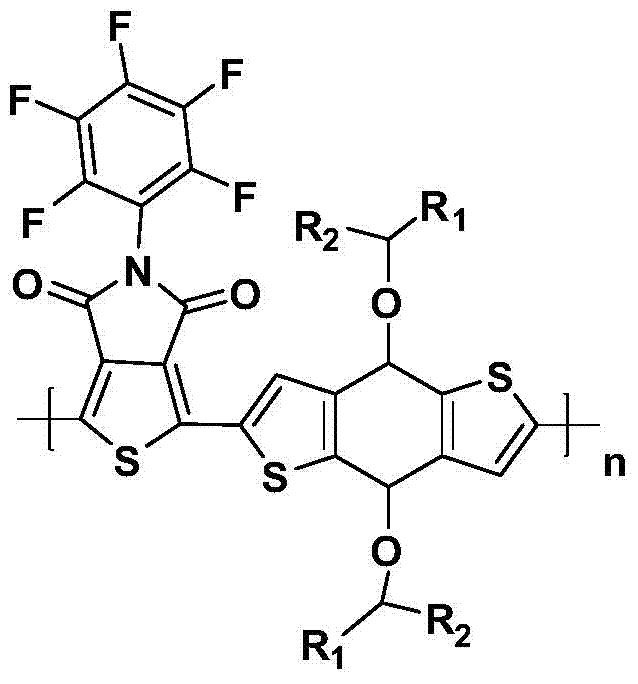
Examples
Embodiment 1
[0049] Preparation of fluorine-containing thienopyrrole diketone monomer
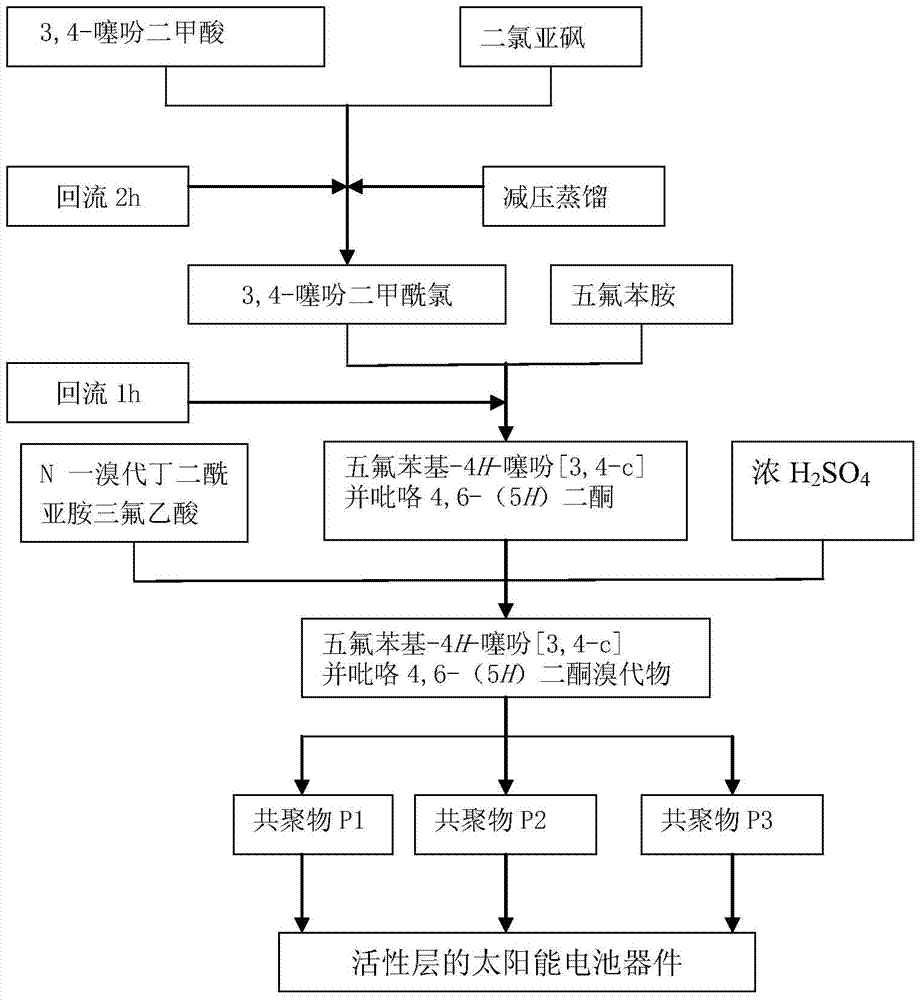
[0050] Mix (1 g, 5.8 mmol) 3,4-thiophenedicarboxylic acid and 10 ml thionyl chloride in an oxygen-free environment filled with inert gas, react at 100°C for 1 hour, cool to room temperature, and distill under reduced pressure The crude product 3,4-thiophenedicarboxylic acid chloride was obtained.
[0051] In an oxygen-free environment filled with inert gas, the obtained crude product 3,4-thiophenedicarboxylic acid chloride and anhydrous toluene were dissolved by heating, and slowly dropped into a solution of pentafluoroaniline (1.17 g, 6.38 mmol) in 3.5 ml of toluene, in Reacted at 120°C for 1 hour, cooled to room temperature, rotary evaporated, extracted, washed, and purified by column chromatography to obtain white crystal pentafluorophenyl-4 H - Thieno[3,4-c]pyrrole 4,6-(5 H ) diketone 0.86g.
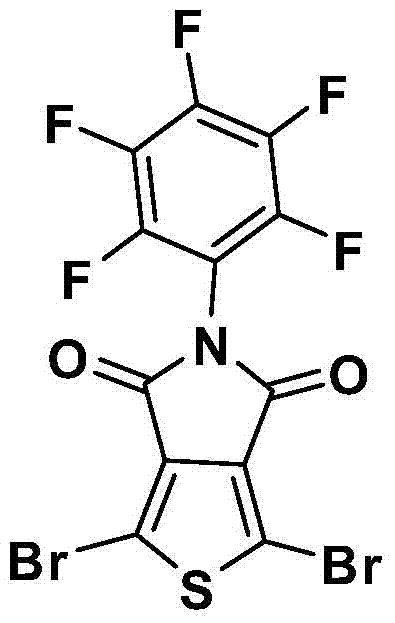
[0052] Take (0.408 g, 1.27 mmol) of pentafluorophenyl-4 H - Thieno[3,4-c]pyrrole 4,6-(5 H ) diketone w...
Embodiment 2
[0054] Preparation of fluorine-containing thienopyrrole diketone monomer
[0055] Mix (2 g, 11.6 mmol) 3,4-thiophenedicarboxylic acid and 30 ml thionyl chloride in an oxygen-free environment filled with inert gas, react at 10°C for 7 h, cool to room temperature, and distill under reduced pressure to obtain Crude product 3,4-thiophenedicarboxylic acid chloride.
[0056] In an oxygen-free environment filled with inert gas, the obtained crude product 3,4-thiophenedicarboxylic acid chloride and anhydrous toluene were dissolved by heating, and slowly dropped into 180 mL of toluene solution of pentafluoroaniline (6.35 g, 34.8 mmol), in Reacted at 10°C for 14 hours, cooled to room temperature, rotary steamed, extracted, washed, and purified by column chromatography to obtain white crystal pentafluorophenyl-4 H - Thieno[3,4-c]pyrrole 4,6-(5 H ) diketone 1.96 g.
[0057] Take (1.2 g, 3.76 mmol) of pentafluorophenyl-4 H - Thieno[3,4-c]pyrrole 4,6-(5 H ) diketone was added to 40 mL ...
Embodiment 3
[0059] Preparation of fluorine-containing thienopyrrole diketone monomer
[0060] Mix (1.2g, 7.0 mmol) 3,4-thiophenedicarboxylic acid and 10ml thionyl chloride in an oxygen-free environment filled with inert gas, react at 50°C for 14 hours, cool to room temperature, and distill under reduced pressure to obtain Crude product 3,4-thiophenedicarboxylic acid chloride.
[0061] In an oxygen-free environment filled with inert gas, heat and dissolve the obtained crude product 3,4-thiophenedicarboxylic acid chloride and anhydrous toluene, slowly drop into (1.8 g, 10 mmol) pentafluoroaniline toluene solution, at 75 ° C Reaction at room temperature for 7 hours, cooled to room temperature, rotary evaporation, extraction, washing, purification by column chromatography to obtain white crystal pentafluorophenyl-4 H - Thieno[3,4-c]pyrrole 4,6-(5 H ) diketone 1.12 g.
[0062] Take (1.0 g, 3.13 mmol) of pentafluorophenyl-4 H - Thieno[3,4-c]pyrrole 4,6-(5 H ) diketone was added to 16 mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com