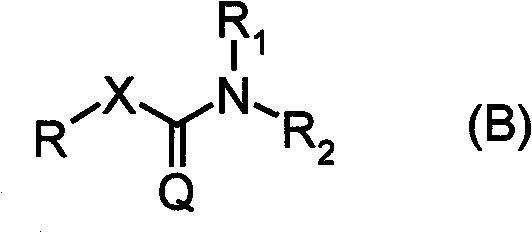
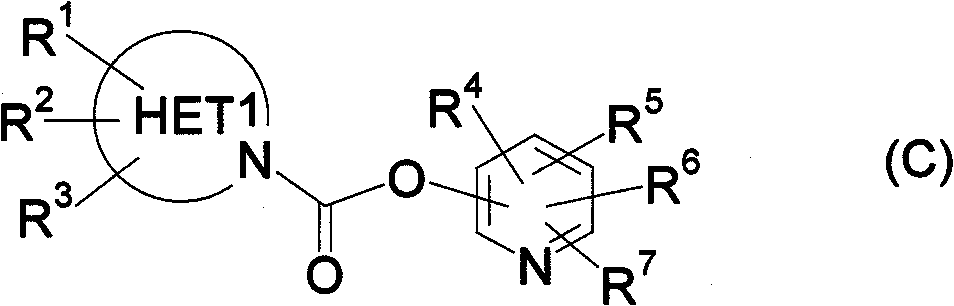
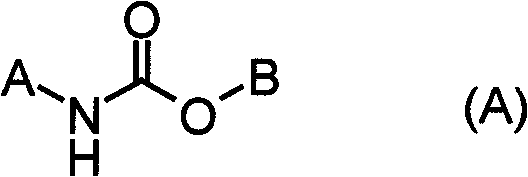Carbamate compound or salt thereof
A technology of compounds and base esters, applied in the field of carbamate compounds or their salts, which can solve the problems of insufficient effect in patients with nocturia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0162] Hereinafter, the production method of the compound of formula (I) is demonstrated in more detail based on an Example. In addition, the present invention is not limited to the compounds described in the following Examples. In addition, the production method of the raw material compound is as shown in the production example. In addition, the production method of the compound of formula (I) is not limited to the production method of the specific examples shown below, and the compound of formula (I) can also be produced by a combination of these production methods or methods obvious to those skilled in the art. manufacture.
[0163] In addition, the following abbreviations may be used in Examples, manufacture examples, and the table|surface mentioned later.
[0164] Pre: Production example number, Ex: Example number, Cpd: Compound number, Str: Structural formula, Syn: Preparation method (indicates the production example number or example number that was produced in the sa...
manufacture example 1
[0166] Add 4-fluorobenzene-1,2-diamine acetic acid (10ml) solution to 2,2,2-methyl trichloroacetimidate (1.53g) in acetic acid (10ml) solution under ice-cooling, at room temperature Stirring was continued for 3 hours. The reaction solution was concentrated under reduced pressure, water was added to the obtained residue, neutralized with saturated aqueous sodium bicarbonate solution, and extracted with EtOAc. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. iPr for residue 2 Recrystallization from O / EtOAc / MeOH afforded 5-fluoro-2-(trichloromethyl)-benzimidazole (1.5 g) as a beige powder. To 1M aqueous sodium hydroxide solution (30 ml) was added 5-fluoro-2-(trichloromethyl)-benzimidazole (500 mg) under ice-cooling, followed by stirring for 1 hour. 1M hydrochloric acid was added to the reaction solution to make it acidic. The resulting solid was filtered off and dried under reduced pressur...
manufacture example 2
[0168] A mixture of tert-butyl piperazine-1-carboxylate (1.75 g), benzimidazole-2-carboxylic acid (1.69 g), HOBt (1.52 g), WSC (1.75 g) and DMF (35 ml) was stirred overnight at room temperature . Saturated aqueous sodium bicarbonate solution was added to the reaction liquid, followed by extraction with EtOAc. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: Hex:EtOAc=2:1 (V / V)) to give 4-(benzimidazol-2-ylcarbonyl)piperazine-1-carboxylic acid as white powder tert-Butyl ester (2.4 g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com