Bufo bufo gargarizans cantor skin extract gelskeleton sustained-release tablets and preparation method thereof
A technology of gel skeleton and extract, applied in the field of preparation and clinical application, can solve the problems of unreachable, long treatment concentration, slow effect and the like, and achieves the advantages of small fluctuation, reduced medication frequency, and less medication frequency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Different models of hypromellose prescription 1, 2, 3 sustained-release tablets (conventional sustained-release tablets):
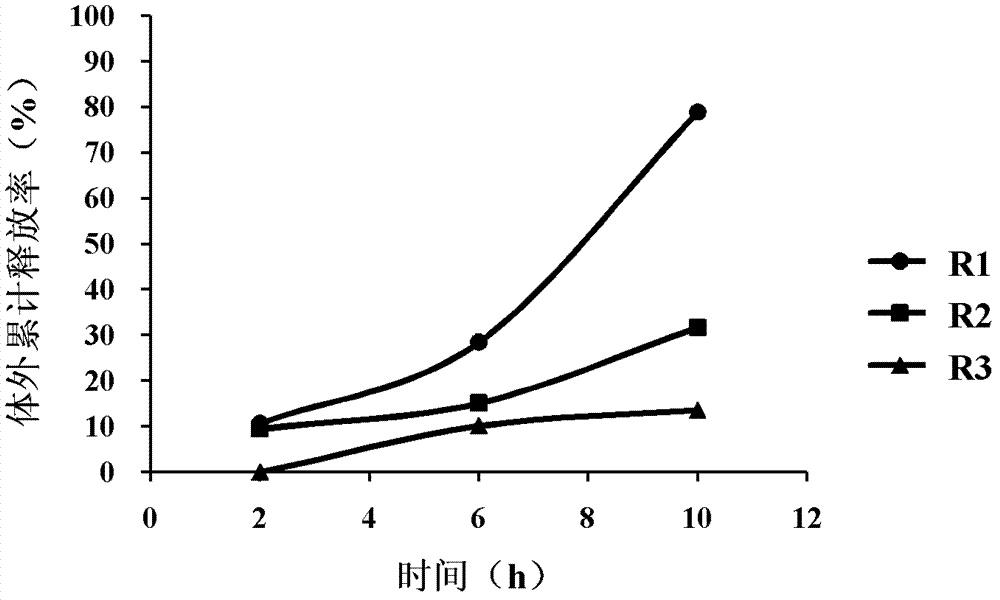
[0041] Weigh the toad skin extract extract, skeleton material (hypromellose K4M, K15M, K100M three types), filler according to the selected prescription ratio, mix them evenly, and crush them through a 60-mesh sieve. Spraying 95% ethanol aqueous solution into wet granulation, after drying the granules, adding an appropriate amount of lubricant talcum powder to the dry granules, mixing evenly, and pressing into tablets to obtain the product. The specific prescriptions and in vitro release conditions of R1, R2, and R3 are shown in Table 1, figure 1 . figure 1 The results showed that the cumulative release in vitro of toad skin extract sustained-release tablets prepared by different types of hypromellose was different. Among them, the cumulative release of active ingredients (ciobufacin base) in the extract of R1 sustained-release tablets increased ...
Embodiment 2
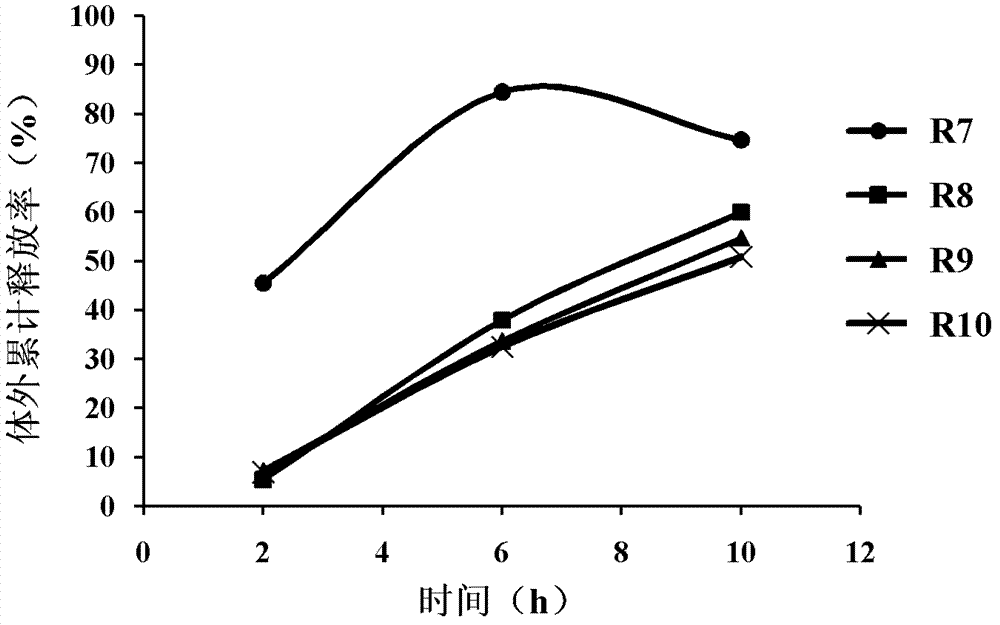
[0045] Prescription R4, R5, R6 of different types of fillers (microcrystalline cellulose, lactose, starch) and prescription R7 of the same type of filler (microcrystalline cellulose) with different dosages (50%, 45%, 40%, 30%) , R8, R9, the preparation method of R10 sustained-release tablet is the same as embodiment 1. See Table 2 for specific prescriptions of R4, R5, R6, R7, R8, R9, and R10.
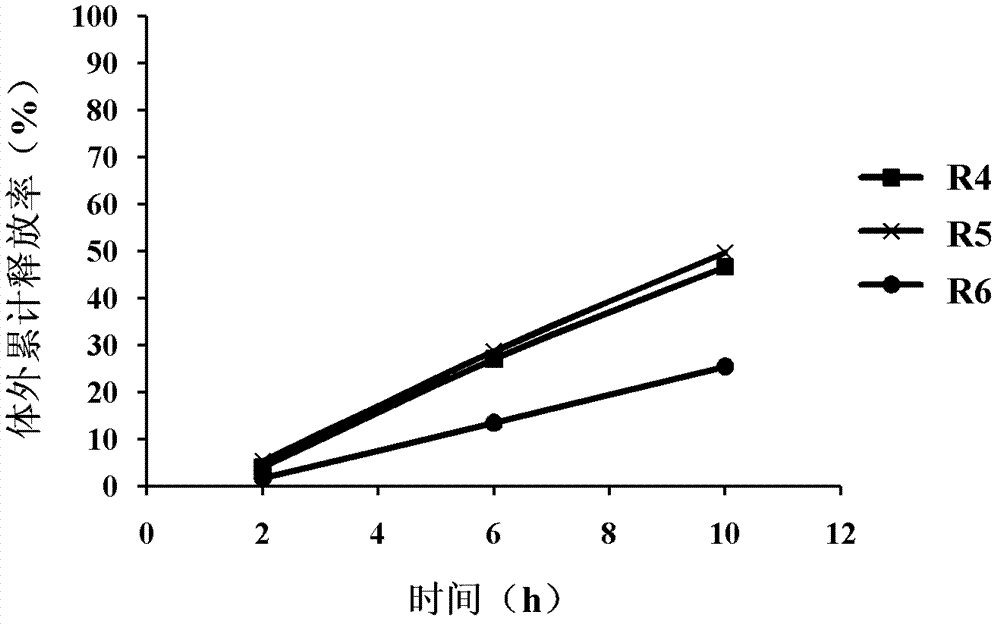
[0046] For the in vitro release of R4, R5, and R6 formulations of different fillers, see figure 2 . Among them, prescription R4 and R5 sustained-release tablets with microcrystalline cellulose and lactose as fillers have a 10-hour cumulative release of active ingredients (ciinobufacin base) in the extracts approaching 50%, and prescription R6 sustained-release tablets with starch as fillers for 10 hours The release of active ingredients in the product is too low. figure 2 The results show that different types of fillers have an impact on the cumulative release of sustained-release ...
Embodiment 3
[0051] Preparation process of prescription 11, 12 sustained-release tablets (different granulation methods):
[0052] Blending and granulation method: crush and sieve the toad skin extract extract and all auxiliary materials separately, mix evenly, spray into appropriate amount of ethanol aqueous solution with a concentration of 95% by volume, wet granulate and press into tablets, and the sustained-release tablets obtained are R11.
[0053] The method of the present invention: take toad skin extract extract powder and auxiliary materials according to the selected prescription ratio, and pulverize and pass through a 60-mesh sieve respectively. The toad skin extract extract powder is divided into Part A and Part B, Part A is mixed with the skeleton material, sprayed into an appropriate amount of ethanol aqueous solution with a volume concentration of 85% for wet granulation, and dried; Part B is mixed with the filler After mixing, spray an appropriate amount of ethanol for wet g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com