Method for preparing nano-gold
A technology of nano-gold and chloroauric acid, which is applied in the field of metal nanoparticle preparation, can solve the problems of uncontrollable, fast reduction, lack of controllability, etc., and achieve the effect of controllable speed and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, the preparation of nano gold
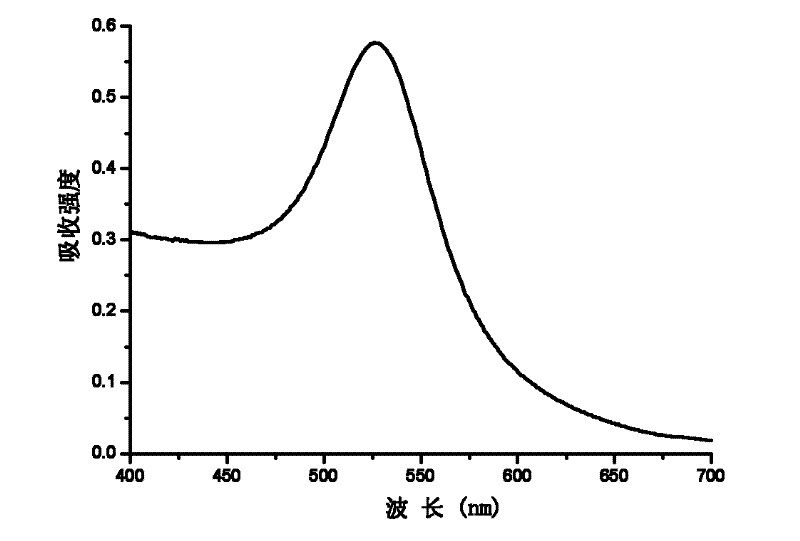
[0023] At 10°C, add 1 mL of glutaraldehyde aqueous solution (containing 20 mg of glutaraldehyde) to 10 mL of chitosan in acetic acid solution (containing 100 mg of chitosan, the average molecular weight of chitosan is 45 kDa), and after reacting for 1 h, add 0.5 mLHAuCl 4 Aqueous solution (containing 5mg HAuCl 4 ), then the mass fraction ratio of chloroauric acid, chitosan and glutaraldehyde is 1: 20: 4, stirs 4h, obtains the red nano-gold solution, records the maximum absorption at 526nm on the ultraviolet-visible spectrophotometer place, such as figure 1 Shown; After treatment, the nano gold can be obtained, and its transmission electron microscope picture is as follows figure 2 As shown, its particle size is 7nm-20nm.
Embodiment 2
[0024] Embodiment 2, the preparation of nano gold
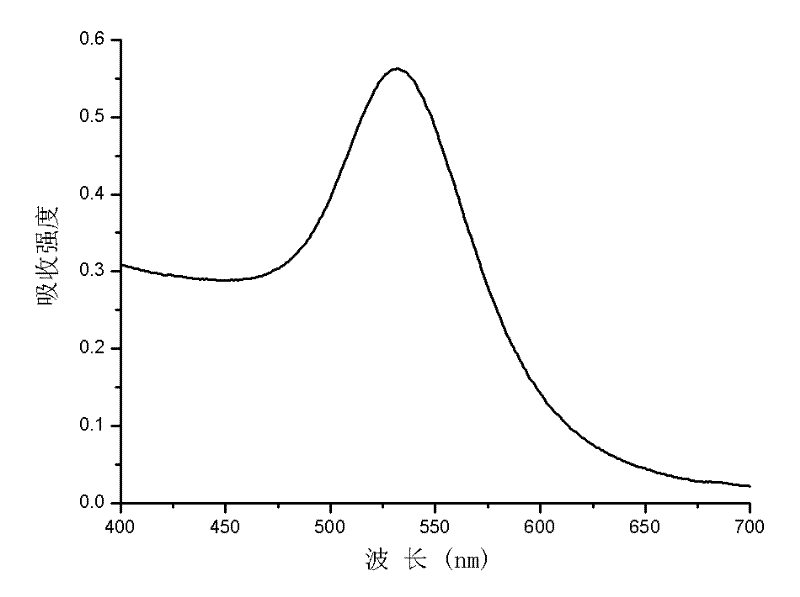
[0025] At 45°C, 0.5mL HAuCl 4 Aqueous solution (containing 5mg HAuCl 4 ) into the acetic acid solution of 10mL chitosan (containing 100mg chitosan, the molecular weight of chitosan is 45kDa), then add 1mL glutaraldehyde aqueous solution (containing 20mg glutaraldehyde), then chloroauric acid, chitosan The mass fraction ratio with glutaraldehyde is 1: 20: 4, stirs 0.5h, obtains the red nano-gold solution, records the maximum absorption at 532nm on the ultraviolet-visible spectrophotometer, as image 3 Shown; After treatment, the nano gold can be obtained, and its transmission electron microscope picture is as follows Figure 4 As shown, its particle size is 6nm-25nm.
Embodiment 3
[0026] Embodiment 3, the preparation of nano gold
[0027] At 25°C, 0.5mL HAuCl 4 Aqueous solution (containing 5mg HAuCl 4 ) into 10mL bovine serum albumin aqueous solution (containing 100mg bovine serum albumin, the molecular weight of bovine serum albumin is 66kDa), then add 1mL glutaraldehyde aqueous solution (containing 20mg glutaraldehyde), then chloroauric acid, bovine serum The mass fraction ratio of albumin and glutaraldehyde is 1: 20: 4, stirs 3h, obtains the red nano-gold solution, records the maximum absorption at 536nm on the ultraviolet-visible spectrophotometer, and the nano-gold particle diameter is 11nm -33nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com