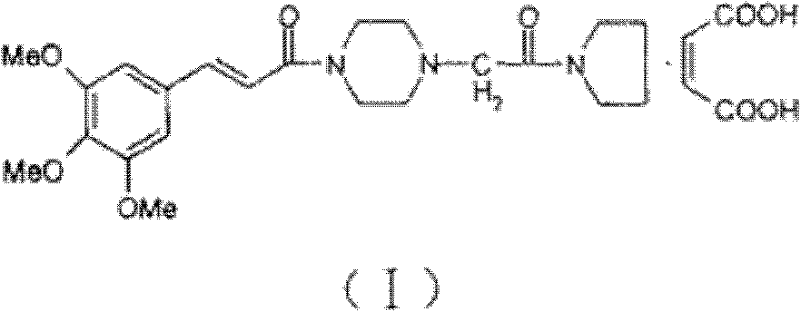
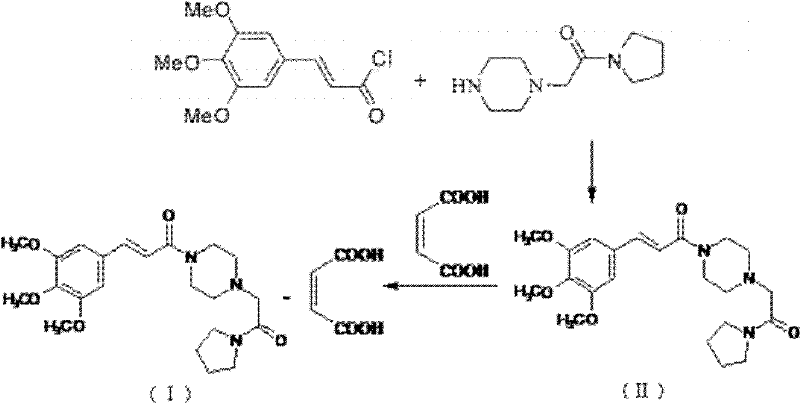
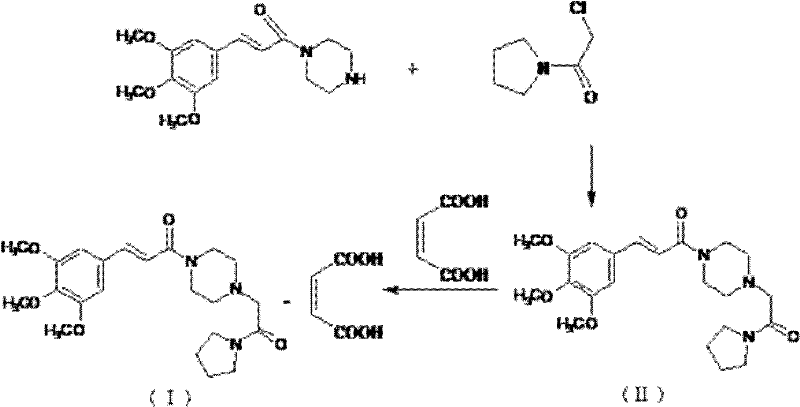
Method for synthesizing cinepazide
A synthesis method and technology of cinnamic acid, applied in the field of medicine and chemical industry, can solve the problems of many by-products, complicated post-processing steps, low yield of reaction products, etc., and achieve the effects of low cost, easy industrial production, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Trans-3,4,5-trimethoxycinnamic acid (40.0g, 0.168mol), 2-chloro-4,6-dimethyl-s-triazine (30.0g, 0.171mol) were stirred and dissolved in 250ml of dichloromethane , slowly add N-methylmorpholine (28.7g, 0.333mol) dropwise under stirring, after 2 hours of heat preservation reaction, filter to obtain cinnamic acid activation product solution; then add 1-[1-(1-pyrrolidinylcarbonyl) Methyl]piperazine (30.0 g, 0.152 mol) was stirred and dissolved in dichloromethane, and the cinnamic acid activator solution was slowly added dropwise under stirring, and the reaction was kept for 5 hours. After the reaction was completed, the reaction solution was washed with 120ml saturated aqueous sodium bicarbonate solution, washed with purified water, and then extracted with dilute hydrochloric acid solution (3mol / L) 100ml*3 times, and the dilute hydrochloric acid layer was adjusted to pH with 20% sodium hydroxide to more than 12, then extract 100ml*3 times with dichloromethane, wash the dich...
Embodiment 2
[0035]Trans-3,4,5-trimethoxycinnamic acid (40.0g, 0.168mol), 2-chloro-4,6-dimethyl-s-triazine (30.0g, 0.171mol) were stirred and dissolved in 250ml of acetone, stirred Slowly add triethylamine (34.0g, 0.334mol) dropwise under heat preservation reaction for 2 hours, filter to obtain cinnamic acid activation product solution; then add 1-[1-(1-pyrrolidinecarbonyl)methyl]piperazine (30.0 g, 0.152 mol) was stirred and dissolved in acetone, and the cinnamic acid activator solution was slowly added dropwise under stirring, and the reaction was kept for 5 hours. After the reaction was completed, the reaction solution was washed with 120ml saturated aqueous sodium bicarbonate solution, washed with purified water, and then extracted with dilute hydrochloric acid solution (3mol / L) 100ml*3 times, and the dilute hydrochloric acid layer was adjusted to pH with 20% sodium hydroxide to more than 12, then extract 100ml*3 times with dichloromethane, wash the dichloromethane layer with 100ml sat...
Embodiment 3
[0037] Trans-3,4,5-trimethoxycinnamic acid (40.0g, 0.168mol), bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride (43.5g, 0.171mol) was stirred and dissolved in 250ml In dichloromethane, diisopropylethylamine (43.05g, 0.333mol) was slowly added dropwise under stirring, and after heat preservation for 2 hours, it was filtered to obtain a cinnamic acid activation product solution; then 1-[1-(1- Pyrrolidinecarbonyl)methyl]piperazine (30.0g, 0.152mol) was stirred and dissolved in dichloromethane, and the cinnamic acid activator solution was slowly added dropwise under stirring, and the reaction was kept for 5 hours. After the reaction was completed, the reaction solution was washed with 120ml saturated aqueous sodium bicarbonate solution, washed with purified water, and then extracted with dilute hydrochloric acid solution (3mol / L) 100ml*3 times, and the dilute hydrochloric acid layer was adjusted to pH with 20% sodium hydroxide to more than 12, then extract 100ml*3 times with dichlorom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com