Chemical synthesis method of chiral D-phenylalanine
A technology for chemical synthesis of phenylalanine, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of complex process, low optical purity of products, high cost, etc., and achieve simple production process operation and high product quality The effect of high optical purity and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
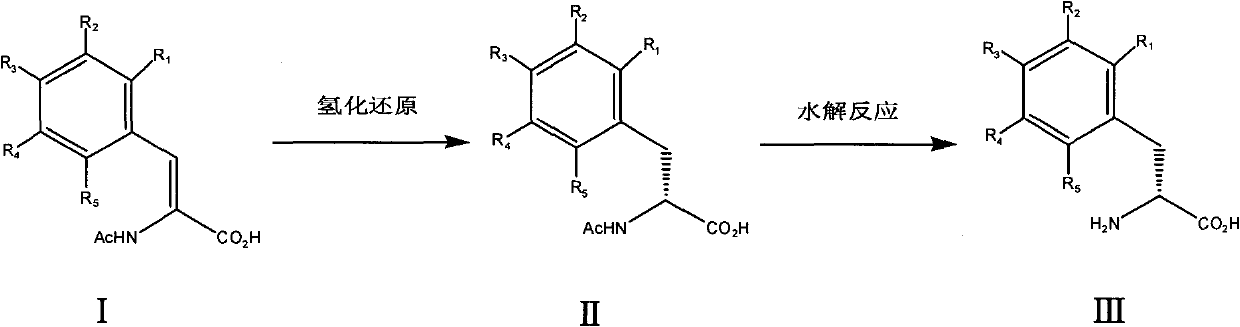
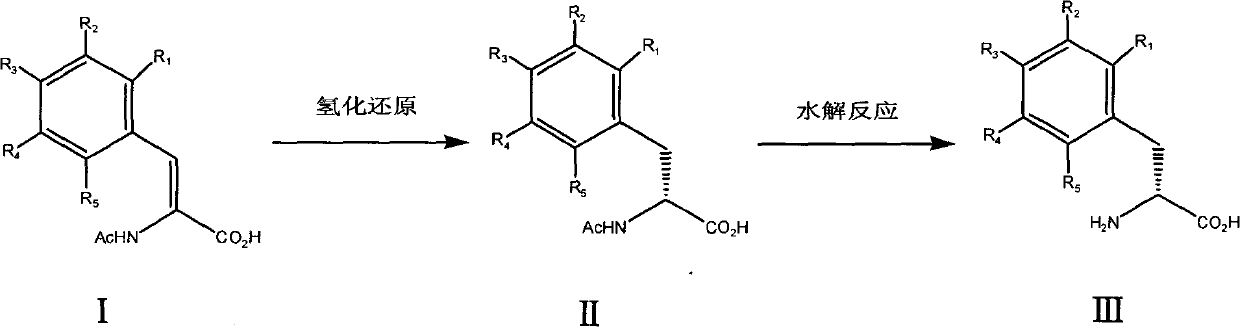
[0016] a): Add 8.2 g of acetamidocinnamic acid ((Z)-2-acetamido-3-phenyl-acrylic acid) and 200 ml of methanol into the autoclave. The reactor was installed and filled with nitrogen. The nitrogen pressure was maintained at 0.7Mpa, and the reactor was stirred for 30 minutes to completely degas the solvent. Then deflate. Add the chiral catalyst ([Rh(1,5-cyclooctadiene)((1R,2R)-bis[(2-methoxyphenyl)phenylphosphine]ethane)]BF4) and a small amount of methanol solution . The reactor was filled with hydrogen, replaced 3 times, and then filled with hydrogen, the pressure was maintained at 1MPa, the internal temperature of the reactor was 30°C, and the reaction was carried out for 8 hours. During the reaction, when the hydrogen pressure was lower than 1MPa, continue to add hydrogen. Then deflate, refill with nitrogen, stir for 30 minutes, release nitrogen, pour out the reaction solution, filter, and evaporate the solvent to dryness to obtain acetamido-D-phenylalanine (II) with a yiel...
Embodiment 2
[0021] a): Add 8.2 g of acetamidocinnamic acid ((Z)-2-acetamido-3-phenyl-acrylic acid) and 200 ml of methanol into the autoclave. The reactor was installed and filled with nitrogen. The nitrogen pressure was maintained at 0.7Mpa, and the reactor was stirred for 30 minutes to completely degas the solvent. Then deflate. The chiral catalyst ([Rh(1,5-cyclooctadiene)((1R,2R)-bis[(2-methoxyphenyl)phenylphosphine]ethane)]BF 4 ) and a small amount of methanol solution were added. The reactor was filled with hydrogen, replaced 3 times, then filled with hydrogen, the pressure was maintained at 3MPa, the internal temperature of the reactor was 30°C, and the reaction was carried out for 5 hours. During the reaction, when the hydrogen pressure was lower than 3MPa, continue to add hydrogen. Then deflate, refill with nitrogen, stir for 30 minutes, release nitrogen, pour out the reaction solution, filter, and evaporate the solvent to dryness to obtain acetamido-D-phenylalanine (II) with a ...
Embodiment 3
[0026] In the same manner as in Example 1, 4-chloro-D-phenylalanine was prepared using (Z)-2-acetylamino-3-(4-chlorophenyl)-acrylic acid raw material. 1 H-NMR (D 2 O), δ2.86-2.97ppm (2H, CH 2 ), 3.91ppm (1H, CH), 7.17ppm (2H, C 6 h 5 ), 7.20ppm (2H, C 6 h 5 ). Yield 81%.
[0027] Product 4-chloro-D-phenylalanine Chemical purity: (HPLC): ≥98.95%
[0028] Optical purity: (HPLC, e.e%): ≥99%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com