Synthetic method of spirocyclic tetronic acid compound key intermediate
A technology of spirocyclic tetronic acid and synthesis method, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., which can solve the problems of hazardous waste, unfavorable industrial production, and excess, so as to reduce energy consumption and environmental pressure , High utilization rate of raw materials, short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
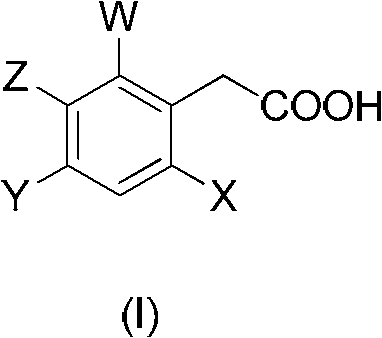
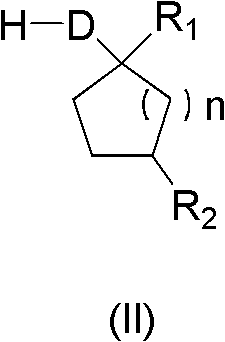
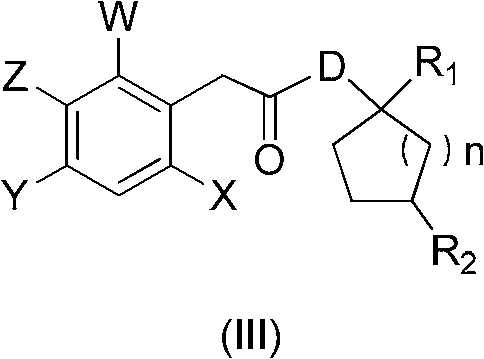
[0023] According to the proportioning in Table 1, add the substituted phenylacetic acid compound (I) and compound (II) into the reactor, heat to the reaction temperature, add the carboxylic acid activating reagent into the reactor, then keep warm for the corresponding time and then cool to room temperature. Then add water to wash, and dry to obtain the corresponding key intermediate compound (III) of the spirocyclic tetronic acid compound. As can be seen from the reaction results in Table 1, the key intermediate compound (III) prepared by the method of the present invention is the key intermediate compound (III) with high yield and high content, and the whole process eliminates the use of organic solvents and is environmentally friendly. ,low cost.
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com