Method for recycling valuable metal in manganese-rich slag
A valuable metal, manganese-rich slag technology, applied in the fields of chemical industry and hydrometallurgy, to achieve the effect of high recovery rate, lower production cost and shorten recovery process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
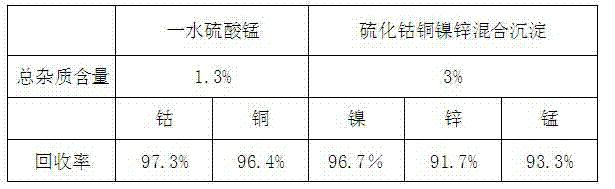
[0015] a leaching 1kg of manganese carbonate slag containing 5% nickel, cobalt, copper and zinc with 5kg 50g / L of sulfuric acid to obtain a sulfate solution containing nickel, cobalt, copper, zinc and manganese;
[0016] b. Crystallize the leached sulfate solution at 180°C for 10 minutes at a constant temperature;
[0017] c Filtrate at 180°C to obtain crystallization mother liquor containing nickel, cobalt, copper and zinc and manganese sulfate monohydrate crystals;
[0018] d Cool the manganese sulfate monohydrate crystals to 50°C to dissolve them by conventional methods, and filter to obtain pure manganese sulfate solution;
[0019] e Recrystallize the pure manganese sulfate solution at a constant temperature for 10 minutes at 180°C, filter and dry at 180°C to obtain manganese sulfate monohydrate crystals;
[0020] f Precipitate the crystalline mother liquor containing nickel, cobalt, copper, and zinc with 120g of sodium sulfide to obtain mixed precipitates of cobalt sulfi...
Embodiment 2
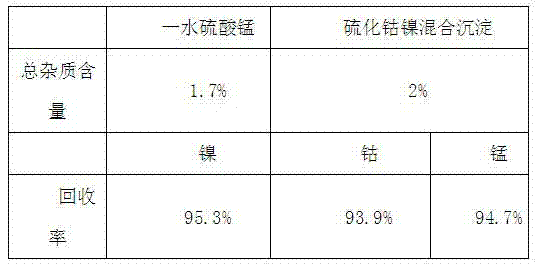
[0025] a Leach 5kg of manganese-rich slag containing 1% nickel and cobalt with 5kg250g / L of sulfuric acid to obtain a sulfate solution containing nickel, cobalt and manganese;
[0026] b. Crystallize the leached sulfate solution at a constant temperature of 250°C for 30 minutes;
[0027] c Filtration at 250°C to obtain crystallization mother liquor containing nickel and cobalt and anhydrous manganese sulfate crystals;
[0028] d Cool anhydrous manganese sulfate crystals to 20°C to dissolve them by conventional methods, and filter to obtain pure manganese sulfate solution;
[0029] e Recrystallize the pure manganese sulfate solution at a constant temperature for 30 minutes at 200°C, filter and dry at 200°C to obtain manganese sulfate monohydrate crystals;
[0030] f Precipitate the crystallization mother liquor containing nickel and cobalt with 330g of sodium sulfide to obtain the mixed sulfide precipitation of nickel and cobalt sulfide, and return the mixed sulfide precipitat...
Embodiment 3
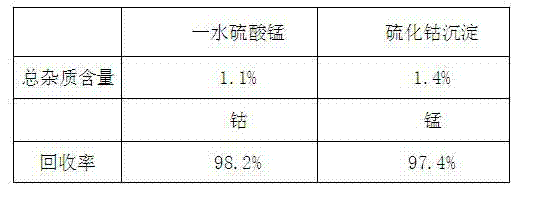
[0035] a Leach 5 kg of manganese-rich slag containing 2% cobalt with 10 kg of 200 g / L sulfuric acid to obtain a sulfate solution containing cobalt and manganese;
[0036] b. Crystallize the leached sulfate solution at 200°C for 25 minutes at a constant temperature;
[0037] c Filtration at 200°C to obtain cobalt-containing crystallization mother liquor and mixture crystals containing manganese sulfate monohydrate and manganese sulfate anhydrous;
[0038] d Cool the mixed manganese sulfate crystals to 40°C to dissolve them, and filter to obtain pure manganese sulfate solution
[0039] e Recrystallize the pure manganese sulfate solution at 195°C for 25 minutes at a constant temperature, then filter and dry at 195°C to obtain manganese sulfate monohydrate crystals;
[0040] f Precipitate the cobalt-containing crystallization mother liquor with 400g of sodium sulfide to obtain a cobalt sulfide precipitate, and return the cobalt sulfide precipitate to the cobalt sulfide ore for re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com