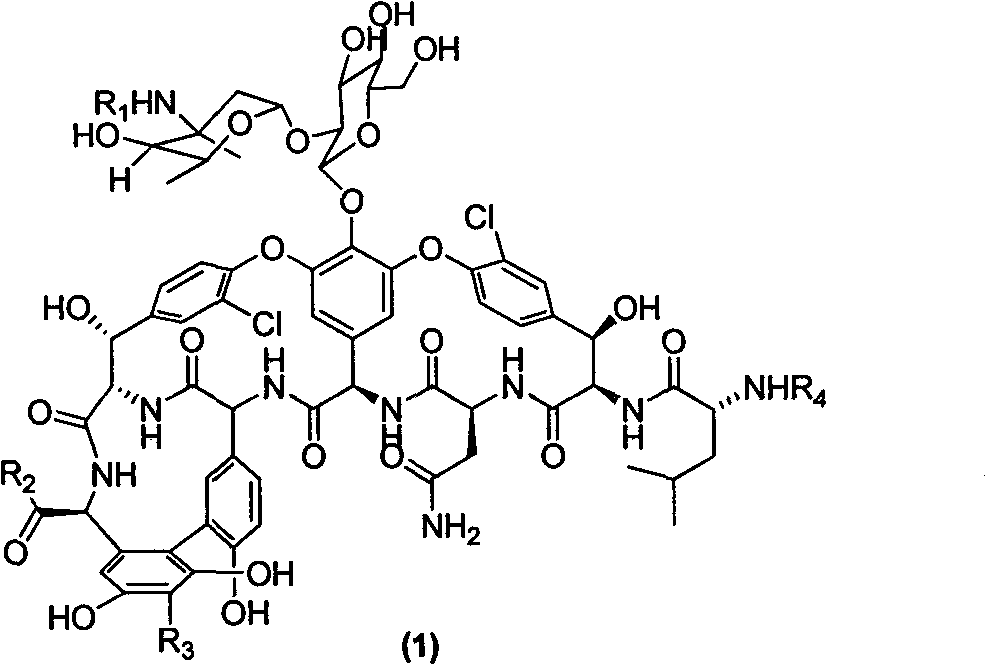
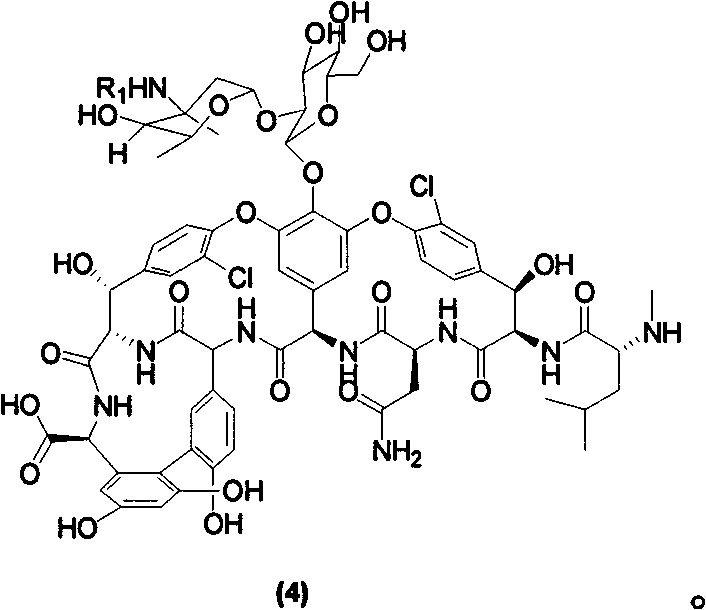
Vancomycin derivate, and preparation method and application thereof
A technology of vancomycin and norvancomycin, applied in the direction of glycopeptide components, antibacterial drugs, peptides, etc., can solve the problems of drug resistance of various microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Synthesis of fluorenylmethoxycarbonyl (Fmoc) norvancomycin
[0040]
[0041] As shown in Scheme 1, add norvancomycin hydrochloride (100mg, 0.068mmol) in a 10ml reaction tube, after dissolving with 0.5ml water and 0.5ml dioxane, add 9-fluouenylmethyl (21.10mg, 0.082 mmol), DIEA (11.65ul, 0.068mmol) was added dropwise, and reacted at room temperature for 10h. The reaction solution was subjected to RP-18 silica gel column chromatography, and the 3 OH:H 2 O=1:1→2:1 gradient elution gave Compound 3110mg with a yield of 95.2%.
Embodiment
[0042] Example Synthesis of 2N-aminocarbonyl vancomycin
[0043]
[0044] As shown in Scheme 2, vancomycin hydrochloride (90mg, 0.061mmol) was added into a 5ml reaction tube, and mixed solvent (Py:H 2 After 2ml of O=1:1) was dissolved, ethyl isocyanate (7.1ul, 0.091mmol) was added dropwise, and reacted at 30°C for 4h. The solvent was evaporated to dryness under reduced pressure, and the residue was purified by reverse-phase silica gel column chromatography with CH 3 OH:H 2 O=1:1 elution gave carboyl vancomycin (4)a 62 mg with a yield of 53%.
[0045] According to the same method, the compounds represented by (4)b-d were prepared in high yields.
Embodiment 3
[0046] Embodiment 3 Synthesis of N-aminocarbonyl norvancomycin
[0047]
[0048] As shown in Scheme 3, the compound N-Fmoc norvancomycin (100mg, 0.06mmol) was dissolved in 2ml of mixed solvent (Py:DMF=1:1), ethyl isocyanate (6.4ul, 0.09mmol) was added dropwise, React at room temperature for 5 hours, evaporate most of the solvent under reduced pressure, add 20ml of diethyl ether to precipitate a white solid, filter off the diethyl ether solution, and then wash the solid with 2*20ml of diethyl ether. The solid was dried to obtain 98.8 mg of the compound with a yield of 94.8%. Add the above solid (90mg, 0.061mmol) into a 5ml reaction tube, dissolve it with 1ml DMF, add dropwise 0.2ml of piperidine, and react at 10°C for 12h. The reaction solution was subjected to silica gel column chromatography, and CHCl 3 :CH 3 OH:H 2 O=4:4:0.7 was eluted to obtain 72 mg of compound (8)a with a yield of 91.8%.
[0049] According to the same method, the compounds represented by (8)b-d we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com