Benzopyran compound and its prepn and use
A technology of benzopyran and compounds, which is applied in the preparation of anti-type II diabetes drugs and the synthesis of benzopyran compounds, which can solve the problems of liver toxicity and high liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
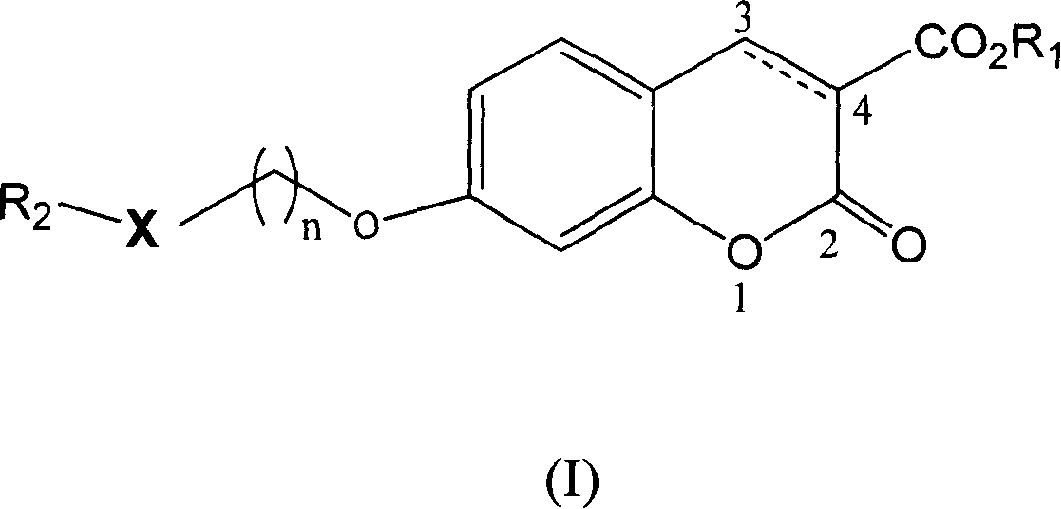
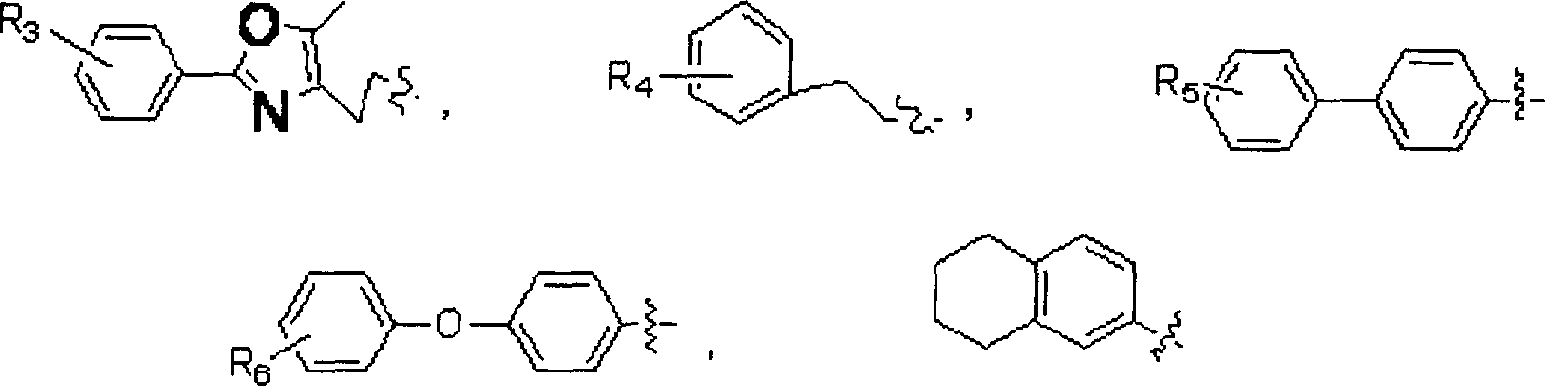
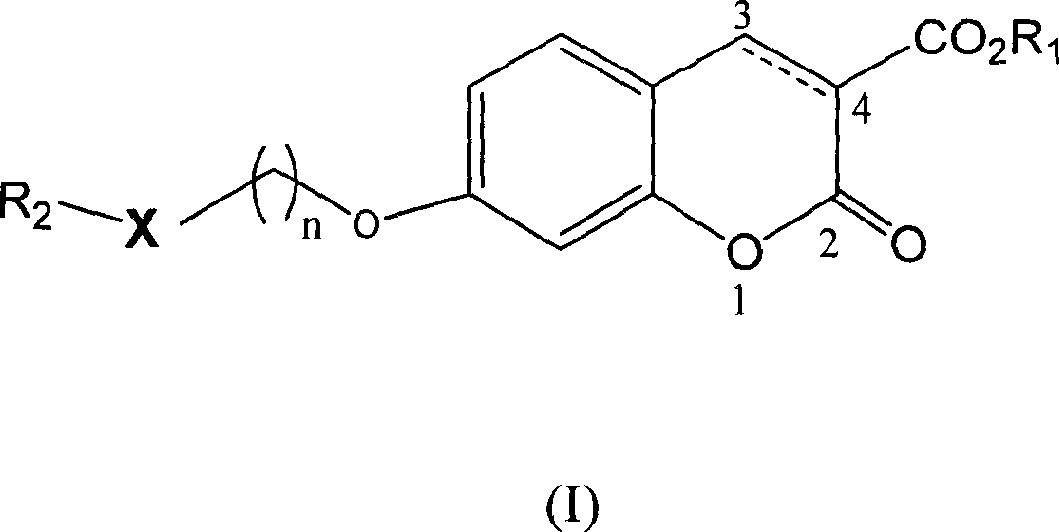
[0086] Example 1: 7-[2-(5-methyl-2-biphenyl-4-oxazole)ethoxy]-2-oxo-2H-1-benzopyran-3-carboxylic acid Esters (compound 1)
[0087] 2-[5-Methyl-2-(4-biphenyl)-4-oxazole]ethanol [Alexander, G.G.; Dawn.A.B.J.Org.Chem.2003, 68, 2623] 0.09g (0.32mmol), Dissolve 0.07g (0.32mmol) of methyl 7-hydroxy-3-coumarincarboxylate in 15ml of anhydrous tetrahydrofuran, add 0.11g (0.42mmol) of triphenylphosphine, and add diethyl azodicarboxylate dropwise at 0°C Ester 0.08ml (0.53mmol). Stir at room temperature for 24 hours. The solvent was distilled off under reduced pressure, and the residue was dissolved in methanol to precipitate a white solid. The resulting solid was recrystallized from methanol to obtain 0.08 g of the target product, yield: 51.6%. M.P.159.0-160.0°C.
[0088] 1 HNMR (CDCl 3 ): δ=2.40(s, 3H), 3.04(t, J=6.3Hz, 2H), 3.94(s, 3H), 4.36(t, J=6.6Hz, 2H), 6.88(m, 2H), 7.37 (m, 5H), 7.60 (m, 4H), 8.04 (dd, J=8.41 Hz, J=6.92 Hz, 1H), 8.54 (s, 1H).
Embodiment 2
[0089] Example 2: 7-[2-(5-methyl-2-(4-biphenyl)-4-oxazole)ethoxy]-3,4-dihydro-2-oxo-4H-1 - Methyl benzopyran-3-carboxylate (compound 15)
[0090] Dissolve 0.07 g (0.14 mmol) of compound 1 in 10 ml of a mixed solvent of methanol:dioxane = 1:3, add 20 mg of 10% Pd-C, mix well, stir and pass hydrogen under normal pressure until hydrogen absorption stops. Pd-C was filtered off, the solvent was distilled off under reduced pressure, the residue was precipitated into a solid in methanol, and 0.06 g of the product was obtained by suction filtration, yield: 85.4%. M.P.122.5-123.0°C.
[0091] 1 HNMR (CDCl 3 ): δ=2.40(s, 3H), 3.03(t, J=6.3Hz, 2H), 3.29(dd, J=15.8Hz, J=6.05Hz, 1H), 3.54(dd, J=15.9Hz, J =8.66Hz, 1H), 3.93(m, 4H), 4.35(t, J=6.7Hz, 2H), 6.86(m, 2H), 7.02(d, J=8.65Hz, 1H), 7.37(m, 5H ), 7.59 (m, 4H).
Embodiment 3
[0092] Example 3: 7-[2-(5-methyl-2-(3-methylphenyl)-4-oxazole)ethoxy]-2-oxo-2H-1-benzopyran- 3-Carboxylic acid methyl ester (compound 2)
[0093] 2-[5-Methyl-2-(3-methylphenyl)-4-oxazole]ethanol 0.40 g (1.84 mmol) [Alexander, G.G.; Dawn.A.B.J.Org.Chem.2003, 68, 2623], Dissolve 0.41g (1.84mmol) of methyl 7-hydroxy-3-coumarincarboxylate in 60ml of anhydrous tetrahydrofuran, add 0.63g (2.40mmol) of triphenylphosphine, and add diethyl azodicarboxylate dropwise at 0°C Ester 0.08ml (3.11mmol). Stir at room temperature for 24 hours. The solvent was distilled off under reduced pressure, and the residue was dissolved in methanol to precipitate a white solid. The resulting solid was recrystallized from methanol to obtain 0.45 g of the target product, yield: 58.3%. M.P.134.0-134.5°C.
[0094] 1 HNMR (CDCl 3 ): δ=2.40(s, 3H), 2.38(s, 3H), 3.02(t, J=6.59Hz, 2H), 3.93(s, 3H), 4.34(t, J=6.59Hz, 2H), 6.83 (d, J=2.33Hz, 1H), 6.88(dd, J=8.66Hz, J=2.34Hz, 1H), 7.22(dd, J=7.70Hz, J=0.69Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com