Matrix metal protease inhibitor and medicinal use thereof
A kind of use, amino technology, applied in the field of new matrix metalloproteinase inhibitors, can solve the problems of low bioavailability, poor selectivity of hydroxamic acids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Synthesis of benzamide ilomastat derivatives
[0019]
[0020] Using the method explored by Liu Keliang et al., using isocaproyl chloride as the starting material to synthesize compound 2 through multiple non-reactions;
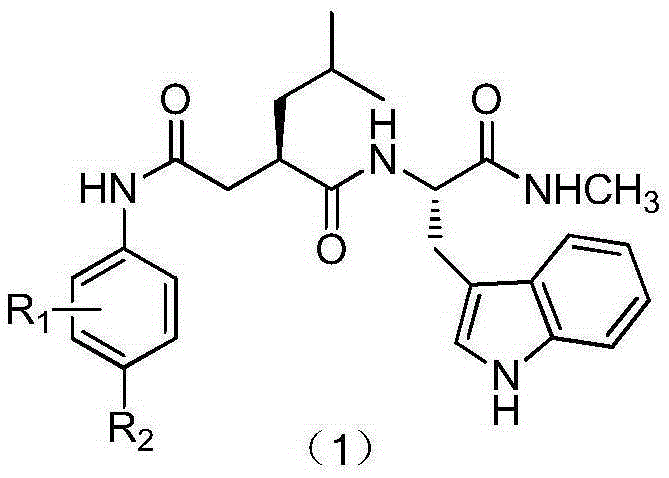
[0021] Compound 1: Take a two-necked bottle, N 2 Protect, vacuumize, first add compound 2, stir and dissolve with anhydrous DCM, add EDCI·HCl and DMAP, react for 1 hour, add phenylenediamine substitute, stir overnight, TLC detection (DCM:MeOH=10:1) raw material Whether the reaction is complete. After the reaction was completed, it was concentrated under reduced pressure, extracted with DCM, saturated NaHCO 3 , water, saturated NaCl solution, and the organic layer was anhydrous NaSO 4 Dry, concentrate, and purify on a silica gel column (DCM:MeOH=30:1-15:1) to obtain the target compound (1a-1h) in powder form.
[0022] N-Deshydroxy-N-(2-aminophenyl)ilomastat (1a)
[0023] White powder, yield 36.23%. 1 H NMR (400MHz, CD 3 OD) δppm: 7....
Embodiment 2
[0038] Example 2: Inhibition of benzamide ilomastat derivatives on MMP-2 and MMP-9
[0039] A fluorescent drug detection kit was used to screen the inhibitory activity of the synthesized compounds on MMP-2 and MMP-9. In this method, a quenched fluorescent peptide is used to screen the corresponding inhibitors. MMP-2 Fluorescent Drug Kit (BML-AK409-0001) and MMP-9 Fluorescent Drug Kit (BML-AK411-0001) (purchased from Enzo Life Sciences company), the screening principle of the kit: quenched fluorescent polypeptide substrate OmniMMP TM fluorgenic substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH 2 [Mca=(7-methoxycoumarin-4-yl)-acetyl; Dpa=N-3-(2,4-dinitrophenyl)-L-α-β-diaminopropionyl]; the fluorescence properties of Mca can be inhibited by the Dpa group, but MMPs can break the quenched fluorescent polypeptide into two chains at Gly-Leu, so that the fluorescence of Mca can be visualized, and then the screening of MMPI inhibitors can be carried out;
[0040] Pretreatment: first tha...
Embodiment 3
[0047] Embodiment 3: the mensuration of the fat-water partition coefficient logP of compound 1a
[0048] Take 20ml of water and 20ml of n-octanol in Erlenmeyer flasks with stoppers (3 copies for each group), vortex for 5min, shake at room temperature for 24h, place in a separating funnel, let stand for 24h, separate the liquid, and take Add about 1.15mg E-1 (1.83mg Ilomastat) to 1ml of the upper layer (saturated n-octanol layer) (so that the concentration of the drug in both n-octanol and aqueous phases is less than 0.01mol / l), and vortex for 5min. Mix with 1ml of n-octanol-saturated aqueous phase, vortex for 5min, shake at room temperature for 24h, centrifuge at 3500rpm for 15min, take 100μL respectively in the sample tube, in 30% acetonitrile + 1% TFA (Ilomastat: 25% acetonitrile + 1% TFA ) under HPLC analysis. Measured: 1a: logP=1.912±0.006; and Ilomastat: logP=1.036±0.005, the results show that benzamide analogue 1a has certain druggability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com