Rosuvastatin calcium oral solid preparation and applications thereof
A technology of rosuvastatin calcium and solid preparations, which is applied in the fields of pill delivery, medical preparations of non-active ingredients, metabolic diseases, etc., which can solve the problems of high cost of preparations, few adverse reactions, low bioavailability, etc. The effects of complete release, avoidance of adverse reactions, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
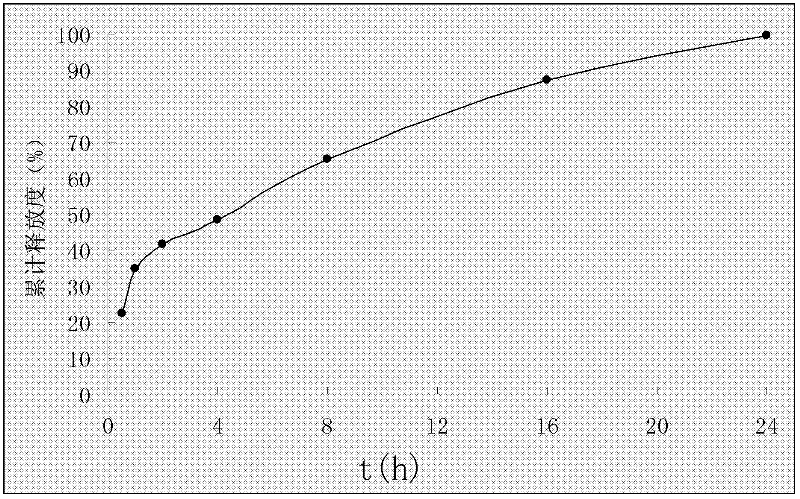
[0034] Example 1 Preparation of Rosuvastatin Calcium Solid Dispersion
[0035] Combine 42g polyethylene glycol 6000 and 59g Add S100 (commercialized polyacrylic acid resin) into 350ml chloroform successively, stir until dissolved, add 20g of rosuvastatin calcium dissolved in a small amount of chloroform while stirring, rotary evaporate below 45°C, evaporate the chloroform and quickly pour into the culture medium Spread it flat in a dish, place it in the freezer layer of the refrigerator for 6 hours, then freeze-dry it for 24 hours, wait for embrittlement, take it out and pulverize it and pass it through an 80-mesh sieve to obtain the solid dispersion of rosuvastatin calcium.
Embodiment 2
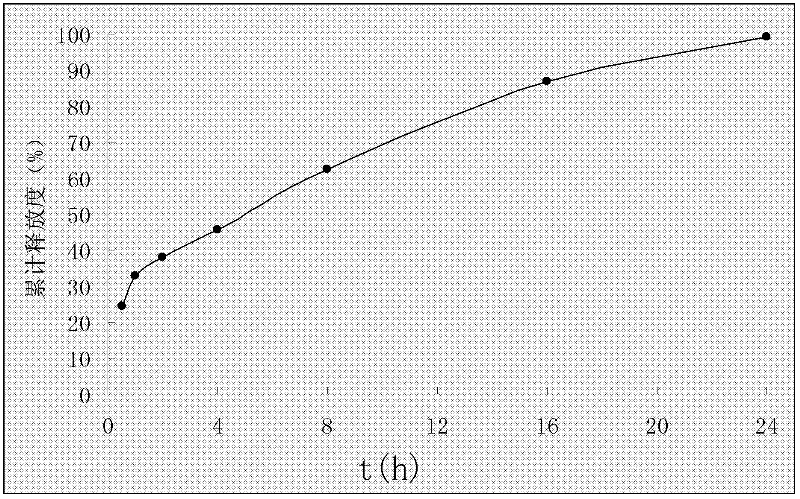
[0036] Example 2 Preparation of Rosuvastatin Calcium Solid Dispersion
[0037] 58g polyethylene glycol 6000, 76g S100 and 65g Add L100 (commercialized polyacrylic acid resin) into 700ml chloroform successively, stir until dissolved, then add 10g rosuvastatin calcium dissolved in a small amount of chloroform while stirring, rotary evaporate below 45°C, evaporate the chloroform and quickly pour into the culture medium Spread it flat in a dish, place it in the freezer layer of the refrigerator for 6 hours, then freeze-dry it for 24 hours, wait for embrittlement, take it out and pulverize it and pass it through an 80-mesh sieve to obtain the solid dispersion of rosuvastatin calcium.
Embodiment 3
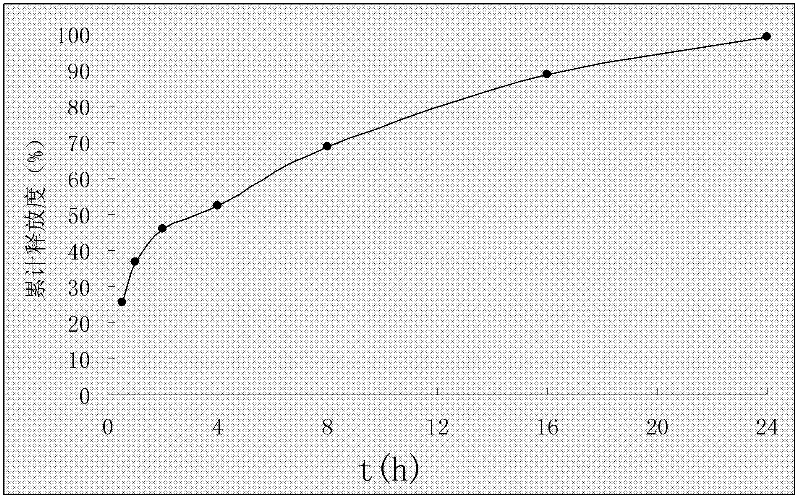
[0038] Example 3 Preparation of Rosuvastatin Calcium Solid Dispersion
[0039] Combine 35g polyethylene glycol 4000 and 28g Add S100 to 300ml chloroform one after another, stir until dissolved, add 20g rosuvastatin calcium dissolved in a small amount of chloroform while stirring, rotary evaporate below 45°C, evaporate the chloroform, quickly pour it into a petri dish, and freeze in the refrigerator The layer is placed for 6 hours, and then freeze-dried for 24 hours. After being embrittled, it is taken out and pulverized and passed through an 80-mesh sieve to obtain a solid dispersion of rosuvastatin calcium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com