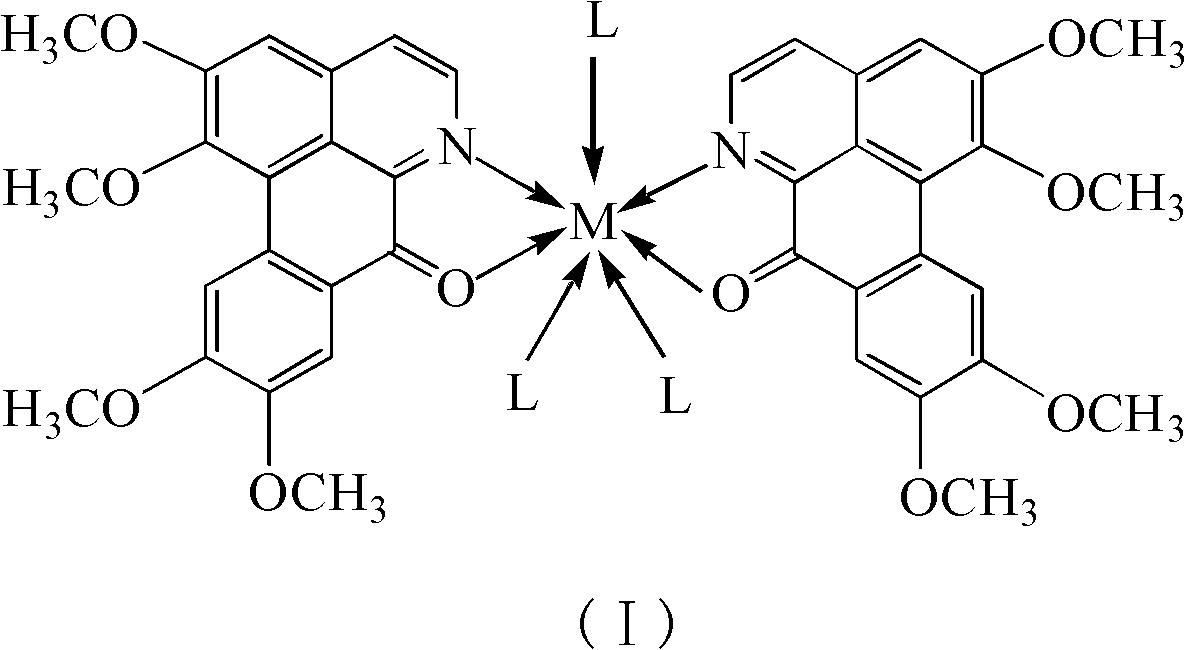
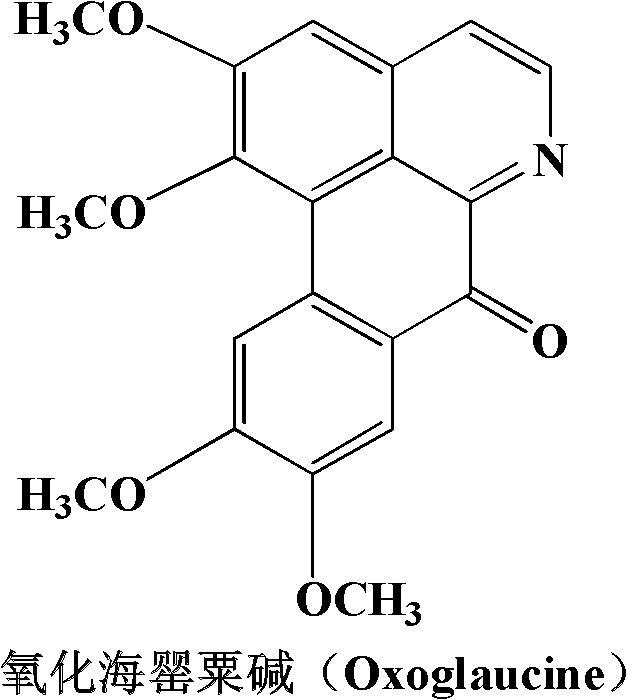
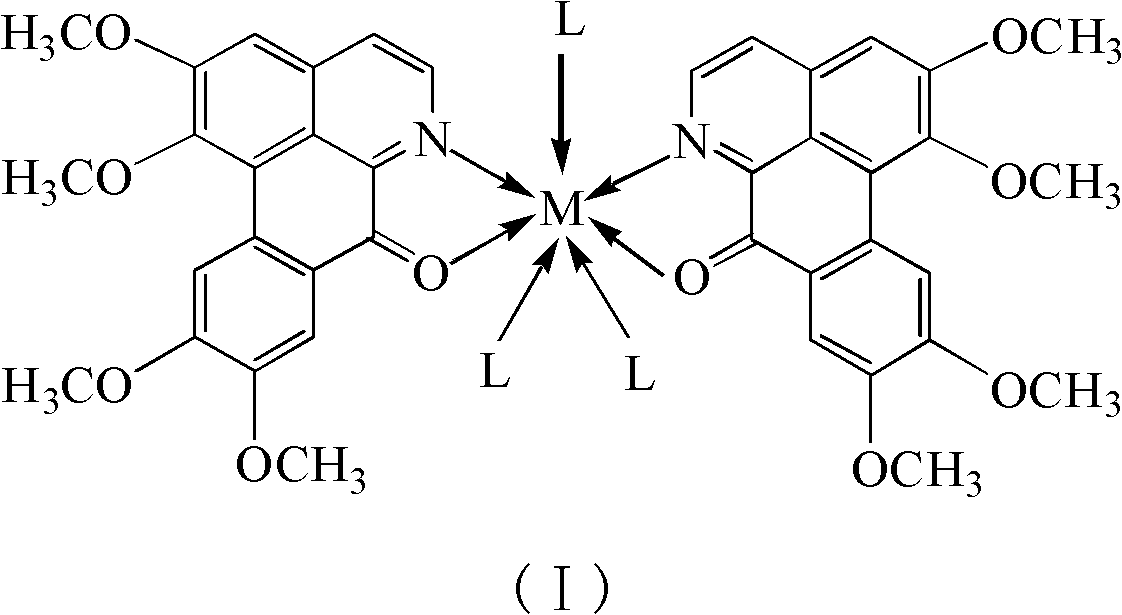
Papaverine rare earth chelate and its synthesis method and application
A technology of rare earth chelate and sea papaverine, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, medical preparations containing active ingredients, etc., to achieve strong anti-tumor activity and good medicine effect with value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Synthesize OGC-La with normal pressure solution method III Chelate
[0029] 1mmol La(NO 3 ) 3 .6H 2 O was dissolved in 5mL of methanol; 2mmol of OGC was heated and dissolved in 15mL of methanol. The two solutions were mixed and reacted at 70°C (reflux for condensation) for 1 hour. After the reaction, a red suspension was obtained with a large number of complexes. A solid was formed; the solution was filtered, and the filtered solid was washed with water, methanol, and ether in turn, and dried to finally obtain a red solid product. The product was determined to be the target chelate [La(OGC) 2 (NO 3 ) 3 ].
Embodiment 2
[0030] Embodiment 2: OGC-Ce is synthesized with normal pressure solution method III Chelate
[0031] 1mmol Ce(Ac) 3 .xH 2 O(Ac represents the acetate anion CH 3 COO - , the same below) was dissolved in 10mL of water; 2mmol of OGC was dissolved in 50mL of ethanol, and the two solutions were mixed and reacted at 80°C (reflux for condensation) for 6 hours. After the reaction, a red suspension was obtained, with a large amount of compound A solid was generated; the solution was filtered, and the filtered solid was washed with water, ethanol, and ether in turn, and dried to finally obtain a red solid product. The product was determined to be the target chelate [Ce(OGC) 2 (Ac) 3 ].
Embodiment 3
[0032] Embodiment 3: OGC-Nd is synthesized with normal pressure solution method III Chelate
[0033] 1 mmol Nd(Ac) 3 .xH 2 O was dissolved in 50mL of water; 2mmol of OGC was dissolved in 150mL of ethanol, and the two solutions were mixed and reacted at 80°C (reflux for condensation) for 24 hours. After the reaction, a red clear solution was obtained; most of them were removed by distillation under reduced pressure Solvent, a large amount of complex solids were precipitated, after cooling, the solution was filtered, and the filtered solids were washed with water, ethanol, and ether in turn, and dried to finally obtain a red solid product. The product was determined to be the target chelate [Nd(OGC) 2 (Ac) 3 ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com