Antibacterial peptide NX-16, and preparation method and application thereof
A technology of NX-16 and antimicrobial peptides, applied in chemical instruments and methods, peptides, bacteria, etc., can solve problems such as unsatisfactory results and affecting the expression of antimicrobial peptides, and achieve easy operation, high antibacterial activity, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Amino acid and gene design of antimicrobial peptide
[0034] (1) The artificially designed antimicrobial peptide NX-16, whose amino acid sequence is ILPWHYPFFPWRRPFR, and PFR is the Kallikrein cleavage site, was artificially synthesized for the entire sequence, and the synthetic peptide was proved to have antibacterial activity by the agarose plate diffusion method, see Example 6.
[0035] (2) Concatenate the new antimicrobial peptide sequence designed in (1) by 3 repeats, design the tandem gene of the tandem amino acid sequence according to the codon preference of Escherichia coli, and add nucleic acid restriction endonucleases at both ends ( xho I and Nco I) Recognition sites and protective bases form the target gene to be expressed.
[0036] The designed tandem target gene sequence is: 5'-G GAATTC A CCATGG ATCCATTTCGTAT TCTGCCGTGGCATTACCCGTTCTTCCCGTGGAGAAGACCATTTCGTATTCTGCCGTGGCATTACCCGTTCTTCCCGTGGAGAAGACCATTTCGTATTCTGCCGTGGCATTACCCGTTCTTCCCG...
Embodiment 2
[0037] Example 2: Primer design and amplification of the target gene
[0038] (1) Using SOEingPCR to design primers, design the target gene described in Example 1 into four complementary overlapping fragments, which contain twelve overlapping base fragments. The overlapping fragments are: G1 contains 57 bases, G2 contains 56 bases, G3 contains 55 bases, and G4 contains 54 bases. Wherein G1 and G2 contain 12 overlapping bases, G2 and G3 contain 17 overlapping bases, and G3 and G4 contain 15 overlapping bases. The base sequences of the four overlapping fragments are:
[0039] G1:G GAATTC A CCATGG ATCCATTTCGTATTCTGCCGTGGCATTACCCGTTCTTCCCGTG
[0040] G2: GGAAGAACGGGTAATGCCACGGCAGAATACGAAATGGTCTTCTCCACGGGAAGAAC
[0041] G3: GCATTACCCGTTCTTCCCGTGGAGAAGACCATTTCGTATTCTGCCGTGGCATTAC
[0042] G4: CCG CTCGAG ACGAAATGGTCTTTCTCCACGGGAAGAACGGGATATGCCACGGCAG
[0043] (2) The two overlapping fragments serve as templates and primers for TD-PCR amplification.
[0044] Use the ov...
Embodiment 3
[0048] Embodiment 3: Construction and identification of expression vector
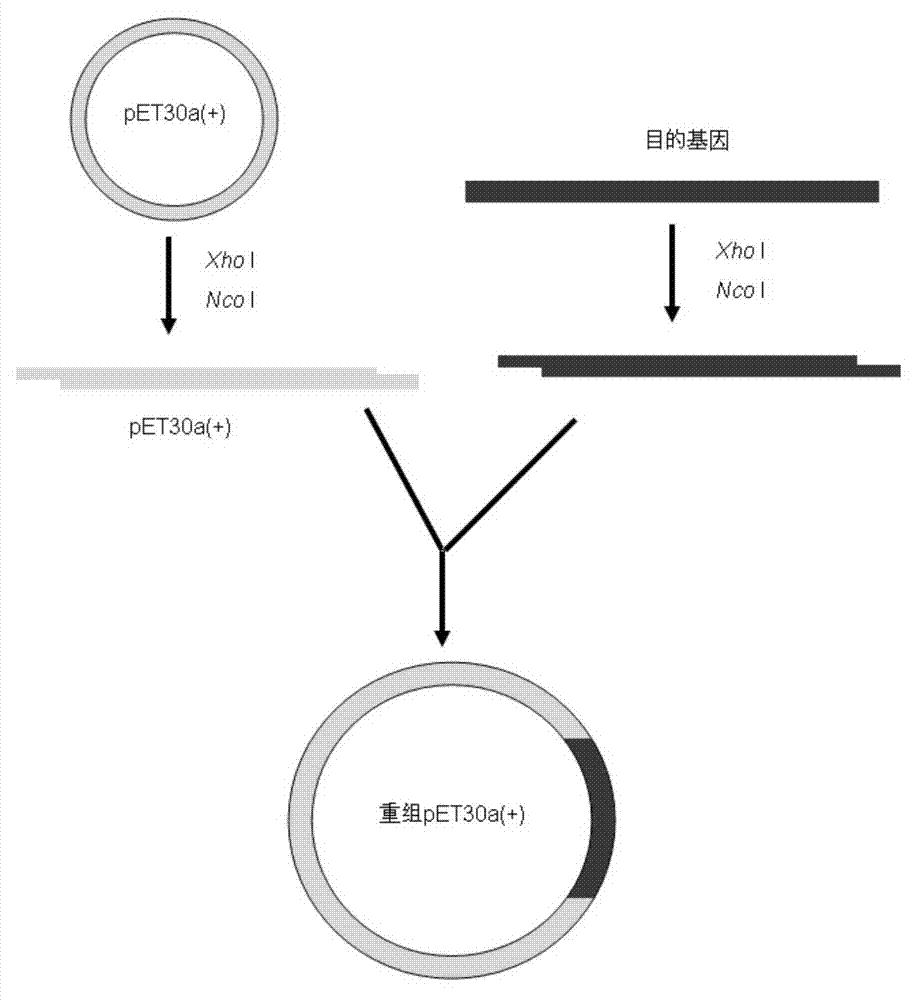
[0049] For the construction method of expression vector, see figure 1 .
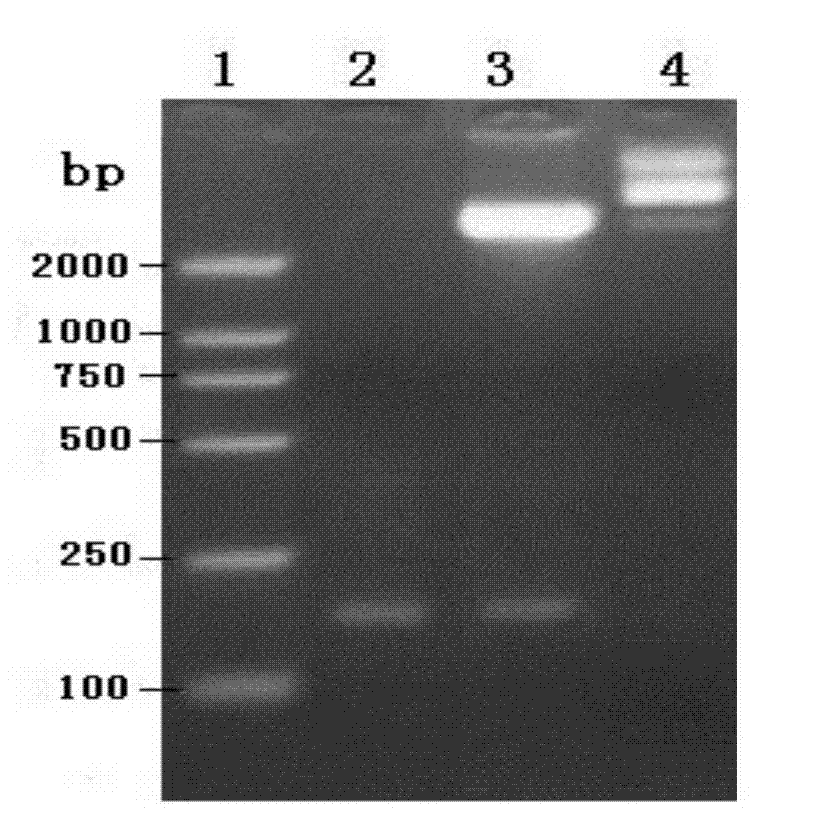
[0050] (1) Extract the plasmid pET30a(+) with a conventional plasmid extraction kit, and then perform double digestion. Enzyme digestion system (50μl): plasmid 30μl, 10×Buffer 10μl, xho I 3 μl, Nco 1 μl of I, 6 μl of deionized water, digested at 37°C for 2 hours, and analyzed by 1% agarose gel electrophoresis.
[0051] (2) Double digestion of the target gene. Enzyme digestion system (50 μl): 30 μl of the target gene obtained in Example 2, 10 μl of 10×Buffer, xho I 3 μl, Nco I 1 μl, deionized water 6 μl. Analyzed by 1% agarose gel electrophoresis after digestion at 37°C for 45 min.
[0052] (3) Connection of target gene and vector. Reaction system (10μl): 4ml of recovered DNA fragments, 1ml of vector recovered after digestion, 0.5ml of T4 DNA ligase (350U / μl), 1ml of ligase buffer, 3.5ml of deionized water, in a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com