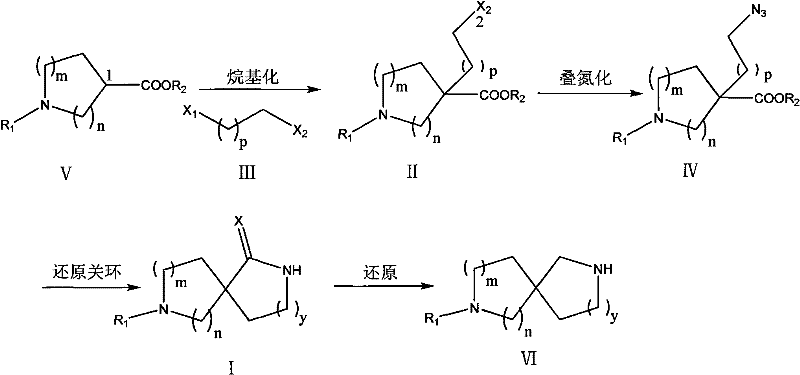
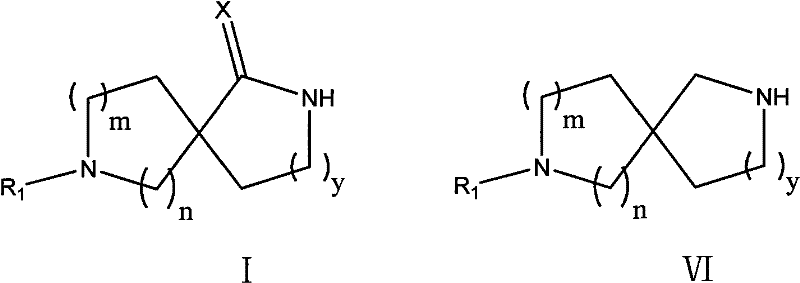
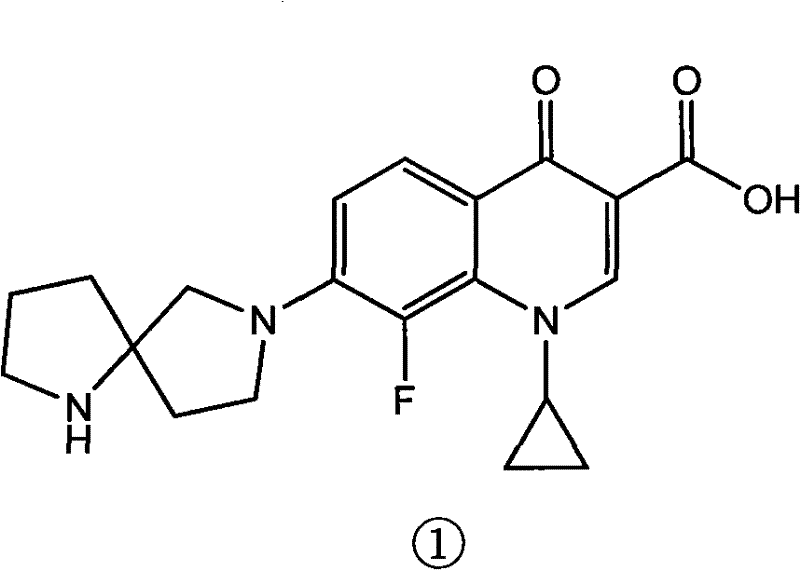
A kind of method for preparing diaza spiro compound
A technology for diazaspiro compounds, applied in the field of preparing diazaspiro compounds, which can solve the problems of expensive reagents, high reaction costs, and low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 (preparation of formula 14 and formula 16)
[0069]
[0070] 1). N 2 For protection, add LDA 300ml (0.6mol, 2.0eq) dropwise to 200ml 4-Boc ethyl piperidinecarboxylate (Formula 11) (77g, 0.3mol) in dry THF (200mL) solution solvent at -15°C, and continue After stirring at low temperature for 1 hour, the reaction solution was added dropwise at -15°C to 100ml of 1-bromo-3-chloropropane (Formula 15) (95.0g, 2.0eq) in dry THF solution, then slowly warmed up to RT and stirred overnight; The next day, ammonium chloride aqueous solution was added for treatment, and after THF was spun off, the aqueous phase was extracted 3 times with ethyl acetate, dried and concentrated to obtain a concentrate;
[0071] 2). Dissolve the concentrate in step 1) in 250ml DMF, and add NaN in batches 3 (40.0g, 2.0eq), N 2 Protect and stir overnight at 50°C to 60°C, extract 3 times with pure petroleum ether the next day, combine organic phases, wash with water, dry and concentrate t...
Embodiment 2
[0079] Embodiment 2 (preparation of formula 24)
[0080]
[0081] 1). N 2 For protection, add LDA 300ml (0.6mol, 2.0eq) dropwise to 4-Boc ethyl piperidinecarboxylate (Formula 21) (77g, 0.3mol) in dry THF (200ml) at -15°C, and continue stirring at low temperature for 1 hour Finally, the reaction solution was added dropwise to 100ml of 1-bromo-2-chloroethane (Formula 25) (86.0g, 2.0eq) in dry THF solution at -15°C, and the temperature was slowly raised to RT and stirred overnight; the next day Ammonium chloride aqueous solution was added for treatment, and after THF was spinned off, the aqueous phase was extracted three times with ethyl acetate, dried and concentrated to obtain a concentrate;
[0082] 2). Dissolve the concentrate in step 1) in 250ml DMF, and add NaN in batches 3 (40.0g, 2.0eq), N 2 Stir overnight at 50°C to 60°C under protection, extract 3 times with pure petroleum ether the next day, combine organic phases, wash with water, dry and concentrate to obtain a...
Embodiment 3
[0086] Embodiment 3 (preparation of formula 34)
[0087]
[0088] 1).N 2 Protection, under the condition of -15°C, dropwise add LDA 300ml (0.6mol, 2.0eq) to the dry THF (200mL) solution of 3-Boc methyl piperidinecarboxylate (Formula 31) (77g, 0.3mol), and continue the low temperature After stirring for 1 hour, the reaction solution was added dropwise to 100ml of 1-bromo-3-chloropropane (Formula 35) (95.0g, 2.0eq) in dry THF solution below -15°C, and the temperature was slowly raised to RT and stirred overnight; The next day, ammonium chloride aqueous solution was added for treatment, and after THF was spinned off, the aqueous phase was extracted three times with ethyl acetate, dried and concentrated to obtain a concentrate;
[0089] 2). Dissolve the concentrate in step 1) in 250ml DMF, and add NaN in batches 3 (40.0g, 2.0eq), N 2 Stir overnight at 50°C to 60°C under protection, extract 3 times with pure petroleum ether the next day, combine organic phases, wash with wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com