Method for preparing 17α-hydroxyprogesterone or its analogues
A technology for hydroxyprogesterone and analogs, which is applied in the field of preparing 17α-hydroxyprogesterone or its analogs, can solve problems such as restricted use, and achieve the effects of improving reaction yield, reducing production cost, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
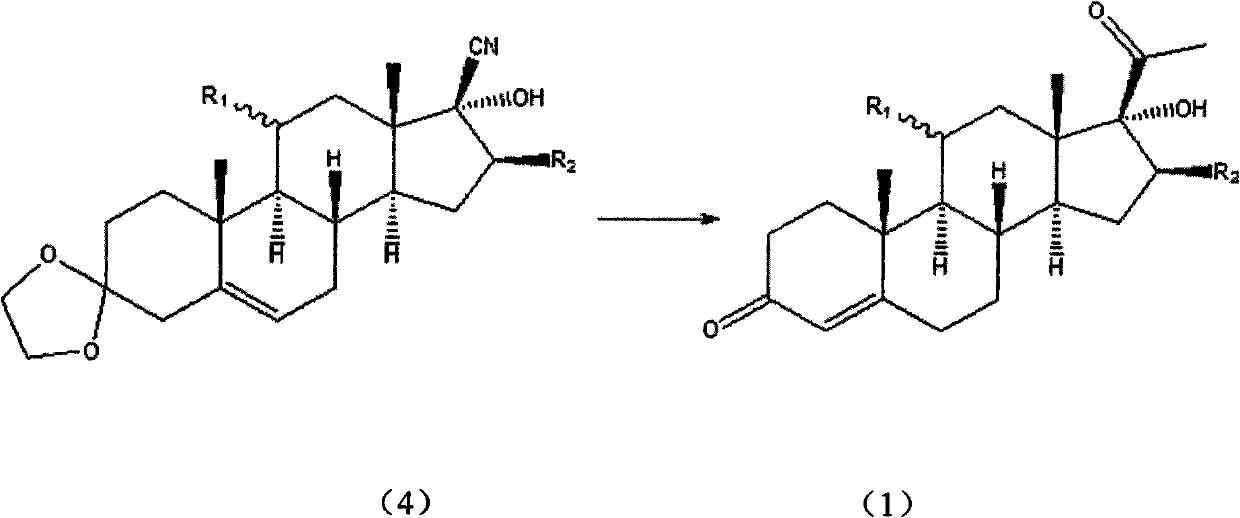
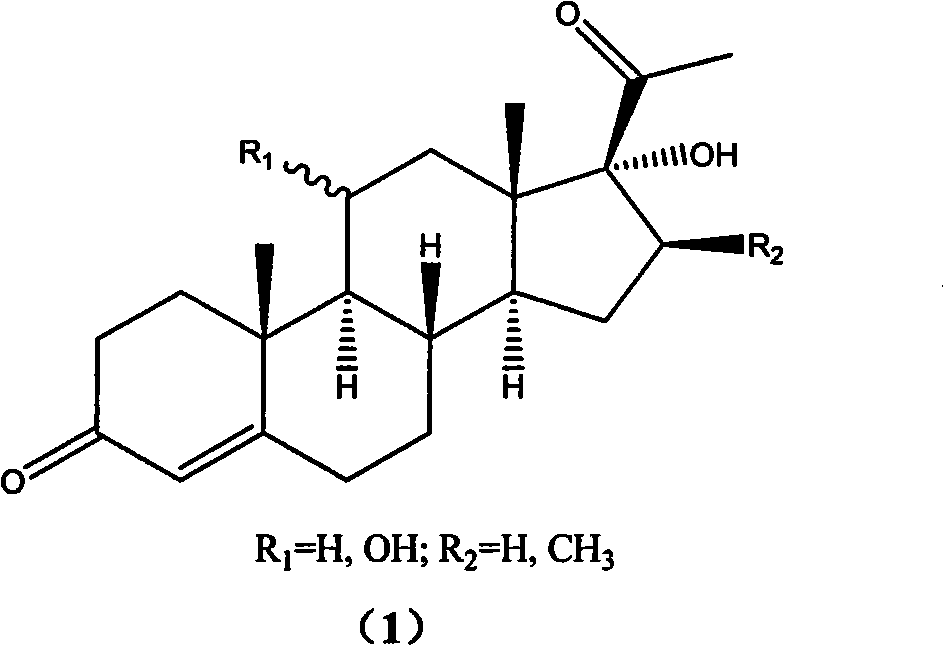
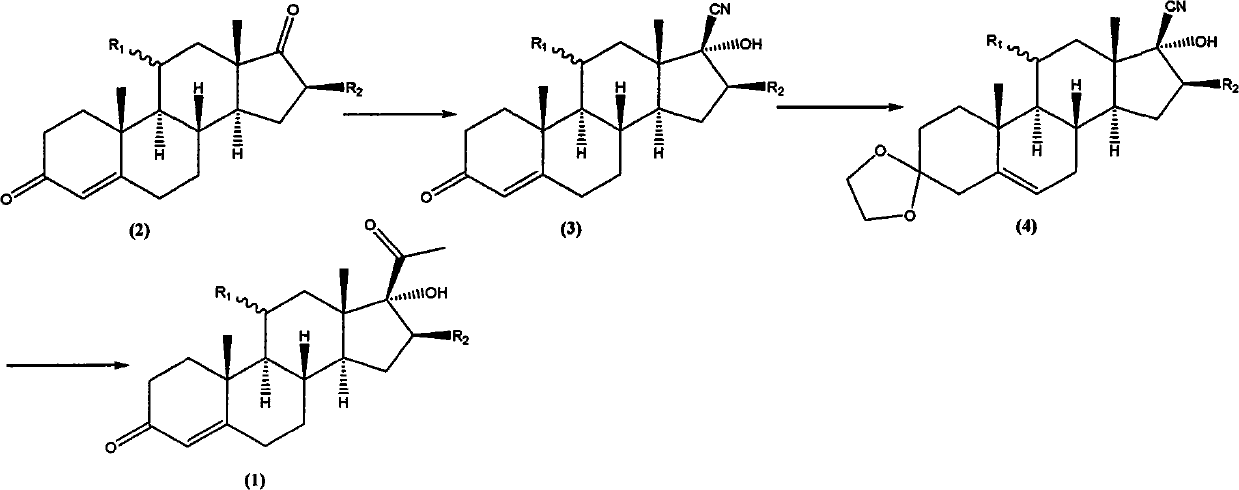
[0033] Preparation of 11α, 17α-dihydroxy-16β-methylpregna-4-diene-3,20-dione (1):
[0034] Add compound (4) (19.28g; Fw: 385.54; 50mmol), magnesium chips (1.34g; Fw: 24.30; 55mmol) successively in a dry 250mL three-necked flask equipped with a thermometer, reflux condenser, and magnetic stirring, without Lithium chloride water (3.18g; Fw: 42.39; 75mmol), 150 ml of tetrahydrofuran; then the temperature of the system was lowered to 10°C, and 75 mL of a 1M solution of tetrahydrofuran in methyl chloride was added dropwise to the reaction system, and the system was maintained during the dropwise addition. At about 20°C, continue to stir for 2h, and the reaction is complete. After the reaction was completed, 50 mL of 2M hydrochloric acid was added dropwise to the reaction system. After the dropwise addition, the temperature was raised to 50° C. and the reaction was continued with stirring for 2 h. After the reaction was completed, the organic phase was separated, and the aqueous ph...
Embodiment 2
[0036] Preparation of 11α, 17α-dihydroxy-16β-methylpregna-4-diene-3,20-dione (1):
[0037] Add compound (4) (19.28g; Fw: 385.54; 50mmol), magnesium chips (1.34g; Fw: 24.30; 55mmol) successively in a dry 250mL three-necked flask equipped with a thermometer, reflux condenser, and magnetic stirring, without Lithium chloride water (5.30g; Fw: 42.39; 125mmol), 150 ml of tetrahydrofuran; then the temperature of the system was lowered to -20°C, and 75 mL of a 1M solution of tetrahydrofuran in methyl chloride was slowly added dropwise to the reaction system. Necessary cooling was performed to maintain the system at about -20°C, and then the stirring was continued for 12 hours, and the reaction was completed. After the reaction was completed, 100 mL of 2M hydrochloric acid was slowly added dropwise to the reaction system. After the dropwise addition, the temperature was raised to 70° C. and the reaction was continued with stirring for 2 h. After the reaction was completed, the organic...
Embodiment 3
[0039] Preparation of 11α, 17α-dihydroxy-16β-methylpregna-4-diene-3,20-dione (1):
[0040] Add compound (4) (19.28g; Fw: 385.54; 50mmol), magnesium chips (1.34g; Fw: 24.30; 55mmol) successively in a dry 250mL three-necked flask equipped with a thermometer, reflux condenser, and magnetic stirring, without Lithium bromide in water (10.86g; Fw: 86.85; 125mmol), 150 ml of tetrahydrofuran; then lower the temperature of the system to -20°C, and slowly add 75 mL of 1M solution of methyl bromide in tetrahydrofuran to the reaction system dropwise, and necessary cooling is required during the dropwise addition , keep the system at about -20°C, then continue to stir for 12h, and the reaction is complete. After the reaction was completed, 100 mL of 2M hydrochloric acid was slowly added dropwise to the reaction system. After the dropwise addition, the temperature was raised to 70° C. and the reaction was continued with stirring for 2 h. After the reaction was completed, the organic phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com