A method for producing caustic soda by purifying brine
A technology for purifying brine and caustic soda, which is applied in the direction of alkali metal hydroxide, etc., can solve the problems of affecting the sodium chloride content of refined brine, reducing the solubility of sodium chloride, and consuming electric energy, so as to reduce steam consumption, production costs, and The effect of power consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
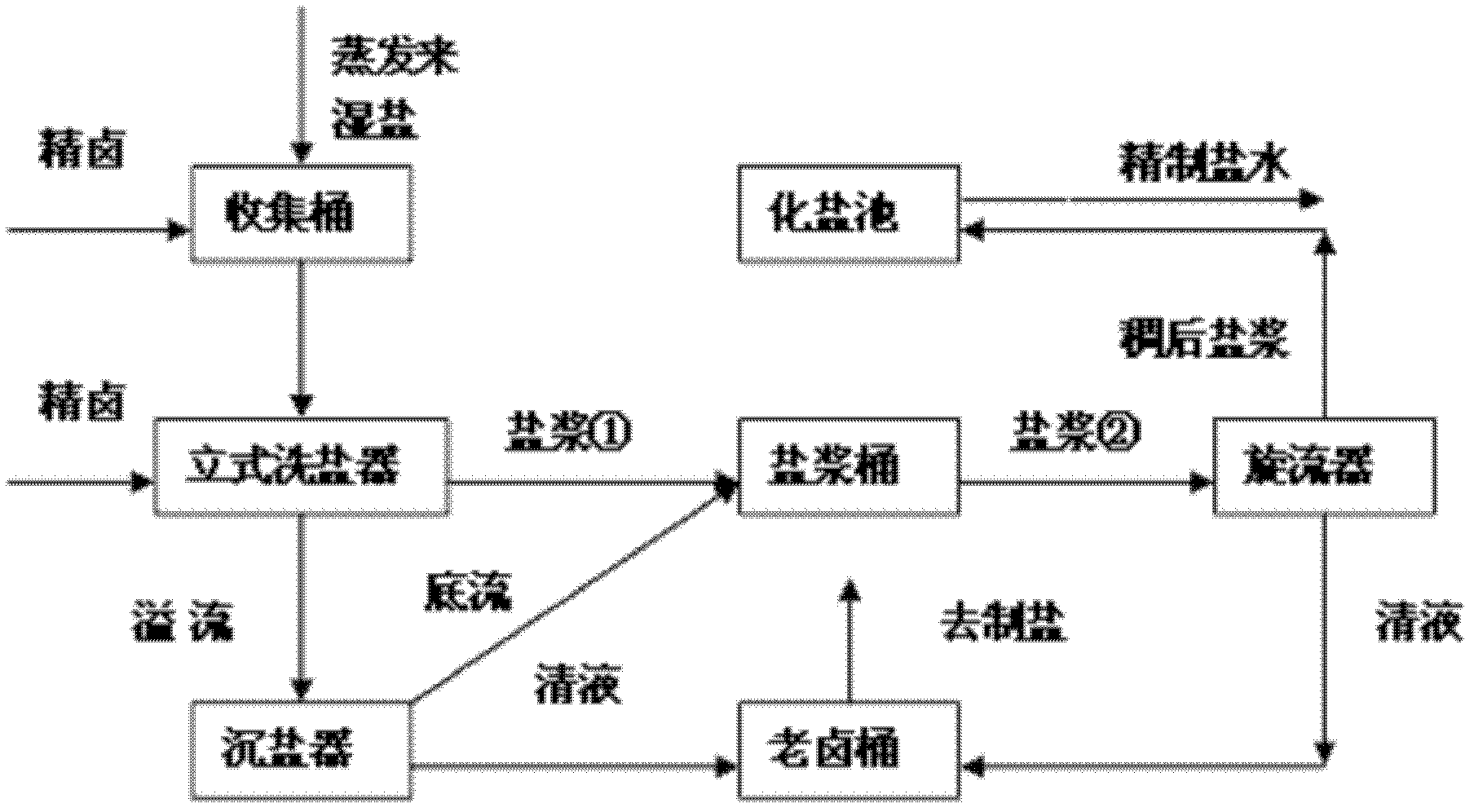
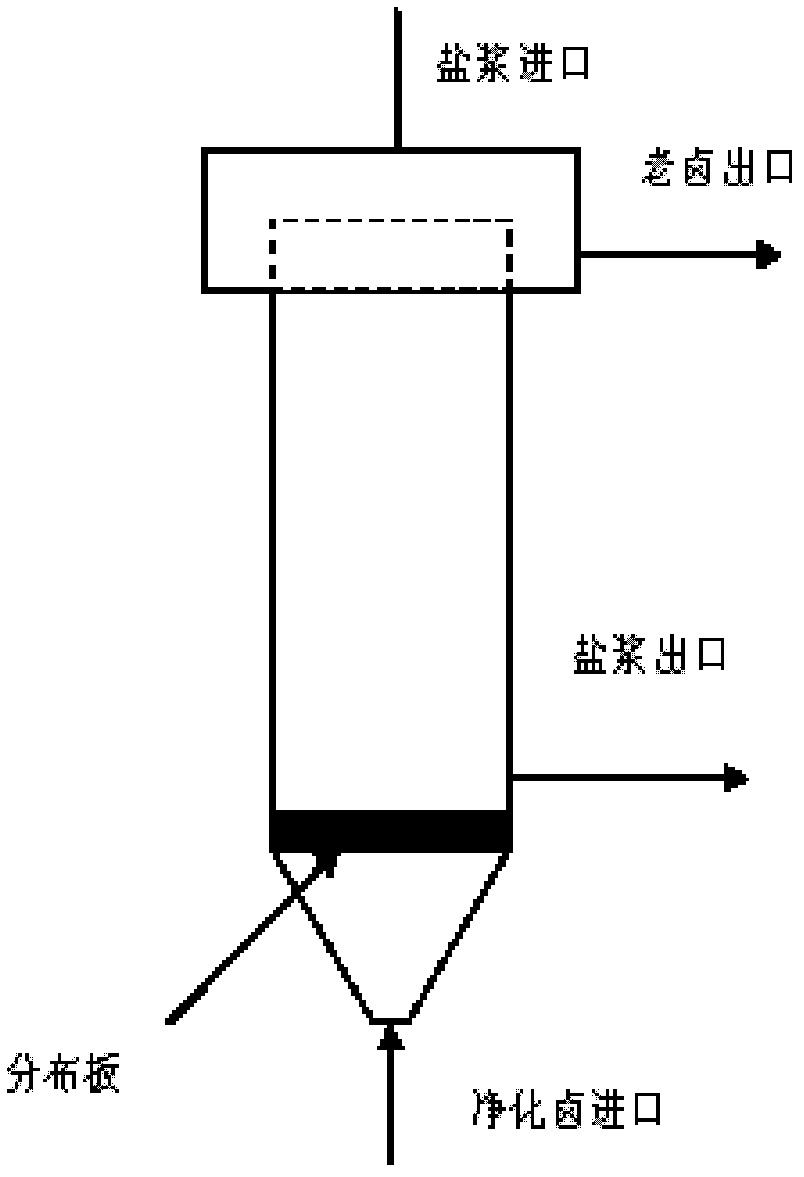
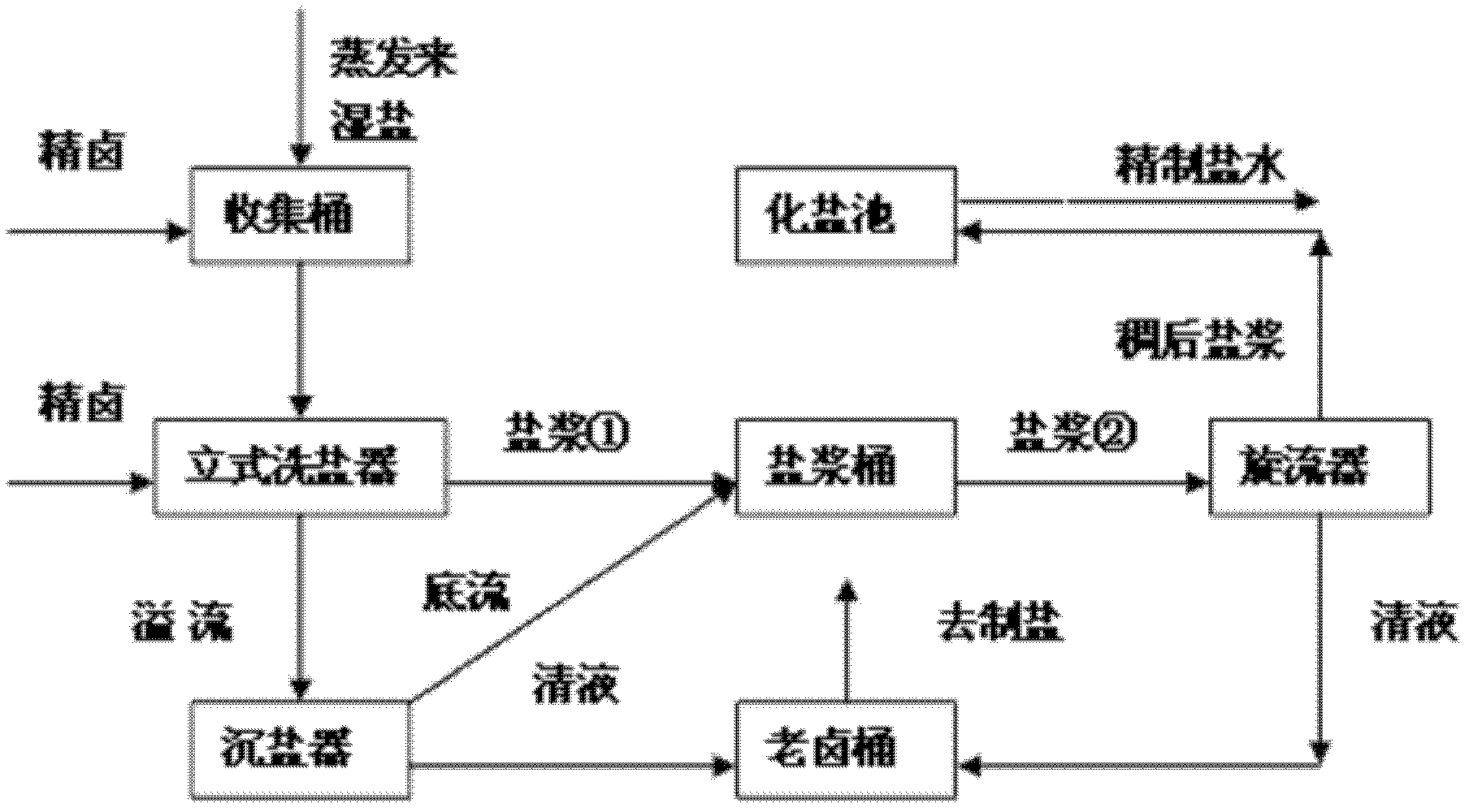
[0010] Below in conjunction with process flow sheet, the present invention is further described:
[0011] Such as figure 1 , figure 2 As shown, the present invention adds a collection barrel, a vertical salt washing device, a salt sinker, an old brine barrel and a cyclone on the recovery salt pipeline separated out in the caustic soda evaporation process, and the salt slurry in the caustic soda evaporation process is collected by the collection barrel Enter the vertical salt washer, and the purified brine (Ca 2+ +Mg 2+ ≤5mg / L) after cooling down (to 32-35°C), redissolving, and flotation, since the sodium sulfate in the purified brine is in an unsaturated state, while the sodium chloride is close to saturation, during the contact washing process, the salt The sulfate radical contained in the slurry will be dissolved in the refined brine, thereby reducing the sulfate radical in the salt slurry and achieving the purpose of purifying the salt quality. The salt slurry is disch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com