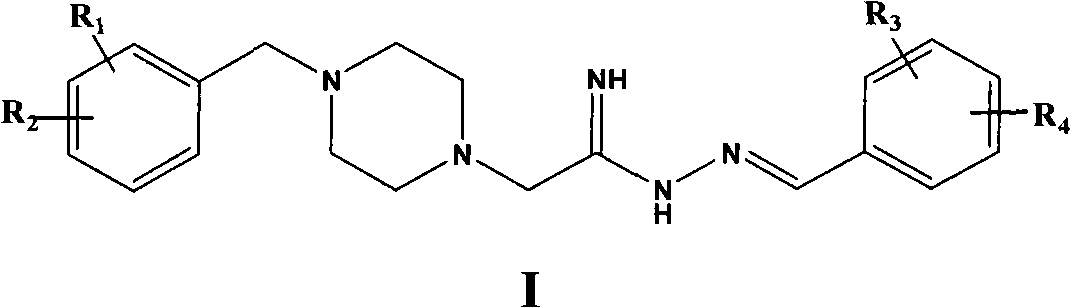
Preparation method of 4-benzylpiperazine ethylimide (iminomethylbenzene) hydrazine compound
A compound and solvate technology, which is applied in the field of synthesis of 4-benzylpiperazine ethyleneimide hydrazide compounds for the treatment of tumors, and can solve problems such as difficulty in purchasing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The synthesis of embodiment 1.4-benzyl piperidine acyl (N-amino iminomethyl-3-hydroxyl phenyl) hydrazine
[0058]
[0059] (1), the preparation of ethyl 2-chloroacetylide hydrochloride:
[0060] In an ice bath, under magnetic stirring, 10 g (130 mmol) of chloroacetonitrile and 6.1 g (130 mmol) of absolute ethanol were dissolved in 100 ml of absolute ether, and dry hydrogen chloride gas was introduced into the reaction solution, and after about 40 minutes A large amount of white solids were precipitated, and the solids were filtered out, washed three times with anhydrous ether, and dried to obtain 16 g of ethyl 2-chloroacetimide hydrochloride, with a yield of 98% and a melting point of 84-86°C. Proton spectrum (400MHz, DMSO): 4.40(s, 2H); 4.20(q, 2H); 1.24(t, 3H).
[0061] (2), the preparation of 2-allyl-6-(N-aminoiminomethyl)phenol:
[0062] At room temperature, under magnetic stirring, add 15ml of methanol, 14.7g (250mmol) of 85% hydrazine hydrate solution in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com