A kind of o-vanillin bisethylenediamine vanadium complex, its synthesis method and its application
A technology of vanadium bis-ethylenediamine and o-vanillin is applied in pharmaceutical formulations, organic chemical methods, preparation of imino compounds, etc., and can solve problems such as aggravating diabetes, tumors, and cell oxidative damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 o-vanillin bisethylenediamine vanadium complex (C 18 h 22 N 2 o 7 V) Synthesis
[0036] (1) Disperse 0.02 mmol of the ligand o-vanillin bisethylenediamine in 1 mL of dimethylformamide to prepare o-vanillin bisethylenediamine solution;
[0037] 0.0020mmol VOSO 4 Disperse in 3mL water to prepare VOSO 4 solution;
[0038] (2) Add o-vanillin bisethylenediamine solution dropwise to VOSO 4 In solution, it is prepared into o-vanillin bisethylenediamine and VOSO 4 The mixed solution; among them, o-vanillin bisethylenediamine and VOSO 4 The molar ratio of the substance is 1:1;
[0039] (3) Put the mixed solution of step (2) in a polytetrafluoroethylene-lined stainless steel reactor, react at 80°C for 72h, then cool down to room temperature at a rate of 5°C / h, and dry naturally to obtain a brown-red Blocky crystals, that is, o-vanillin bisethylenediamine vanadium complex.
Embodiment 2
[0040] Example 2 o-vanillin bisethylenediamine vanadium complex (C 18 h 22 N 2 o 7 V) Structural characterization
[0041] 1. Infrared Spectrum
[0042] Adopt Nicolet NEXUS 470-FTIR type infrared spectrometer to carry out infrared spectrum test to the o-vanillin bisethylenediamine vanadium complex that embodiment 1 obtains (using KBr tablet method, scanning under room temperature, test range is 400~4000cm-1 ). Infrared spectrum characteristic absorption peak (cm -1 ): 3410, 2907, 1637, 1600, 1470, 1440, 1243, 1216.
[0043] 2. Single crystal structure analysis
[0044] Adopt Rigaku Saturn 724 CCD diffractometer to carry out crystal structure test to the o-vanillin bisethylenediamine vanadium complex that embodiment 1 obtains, test result is:
[0045] The vanillin bisethylenediamine vanadium complex is a monoclinic crystal system, P2 1 / n point group; unit cell parameters α=90°, β=93.988(3)°, γ=90°; Z=4; Dc=1.508Mg / m 3 ; I>2σ(I), R 1 =0.0455, wR 2 = 0.1119; al...
Embodiment 3
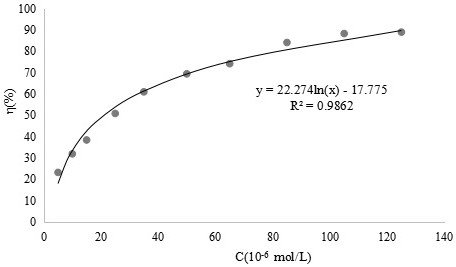
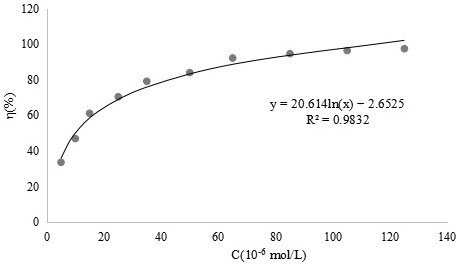
[0048] Example 3 Scavenging effect of o-vanillin bisethylenediamine ligand and its vanadium complex on superoxide anion free radicals
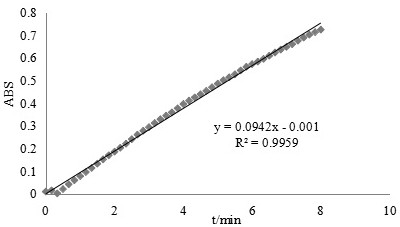
[0049] superoxide anion radical O 2 - It has strong reactivity and short life, so the determination of its activity is not easy to use direct method. Indirect determination of O by pyrogallol autoxidation 2 - clearing effect. Include the following steps:
[0050] (1) Preparation of solution
[0051] Preparation of 0.10mol / L hydrochloric acid solution: accurately pipette 2.25mL of 36.5% concentrated hydrochloric acid into a 250mL volumetric flask, distilled water to volume, and mix well.
[0052] Preparation of 10.0mmol / L hydrochloric acid solution: Accurately pipette 20.0mL of 0.10mol / L hydrochloric acid solution into a 200mL volumetric flask, dilute to volume with double distilled water, and mix well.
[0053] Tris-HCl buffer solution (0.05mol / L Tris, pH=8.20±0.10): Weigh 1.5143g of trishydroxymethylaminomethane, take 57.25mL of 0.10mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com