α-Tetrakis(4-carboxyphenoxy)phthalocyanine and its preparation method and application in the field of molecular self-assembly
A carboxylphenoxy and basephenoxy technology, applied to α-tetra(4-carboxyphenoxy)phthalocyanine and its preparation and application in the field of molecular self-assembly, can solve the problems of low conjugation and the like , to achieve the effects of increased conjugation, good chemical and thermal stability, good solubility and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0010] Specific embodiment one: the structural formula of the α-tetrakis (4-carboxyphenoxy) phthalocyanine of the present embodiment is as follows:
[0011]
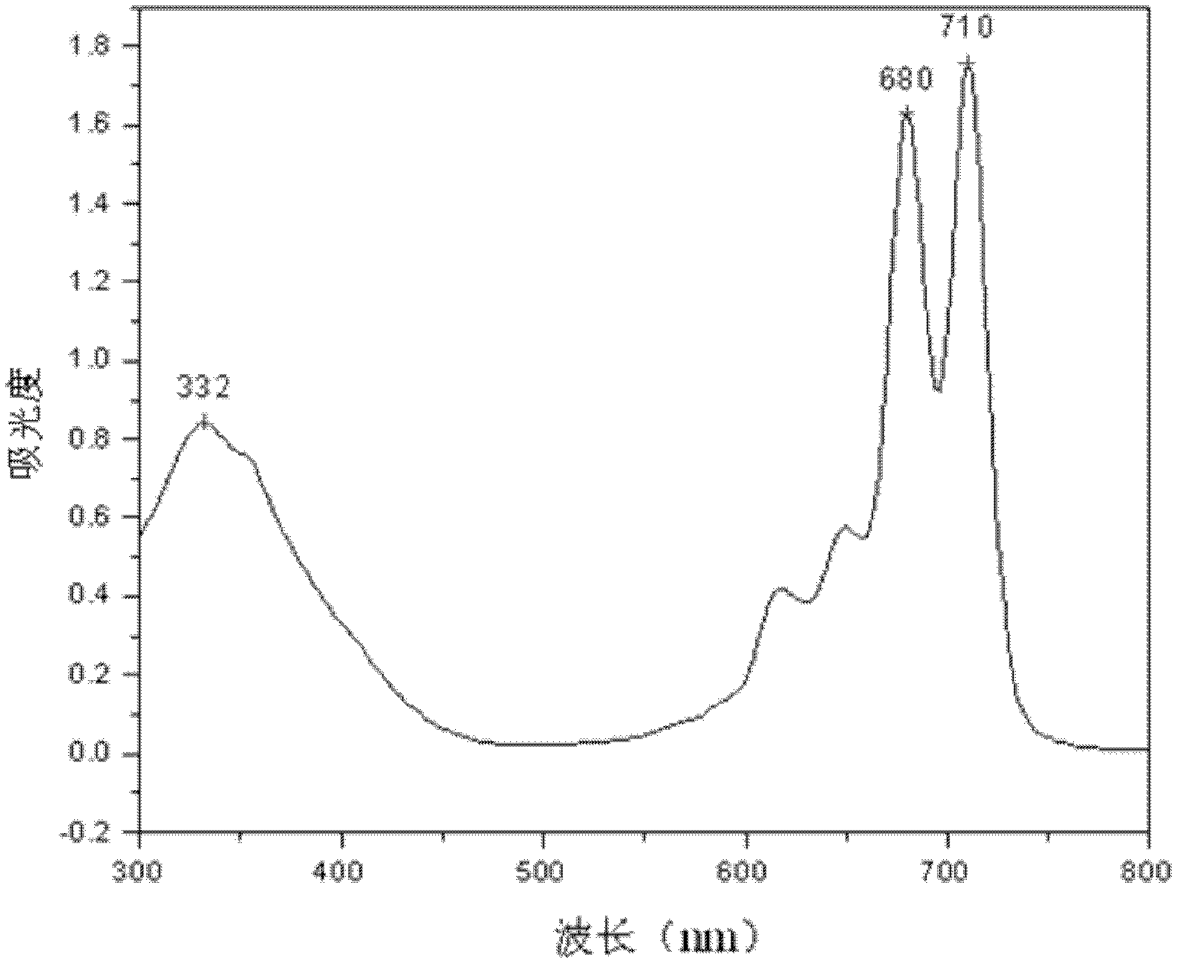
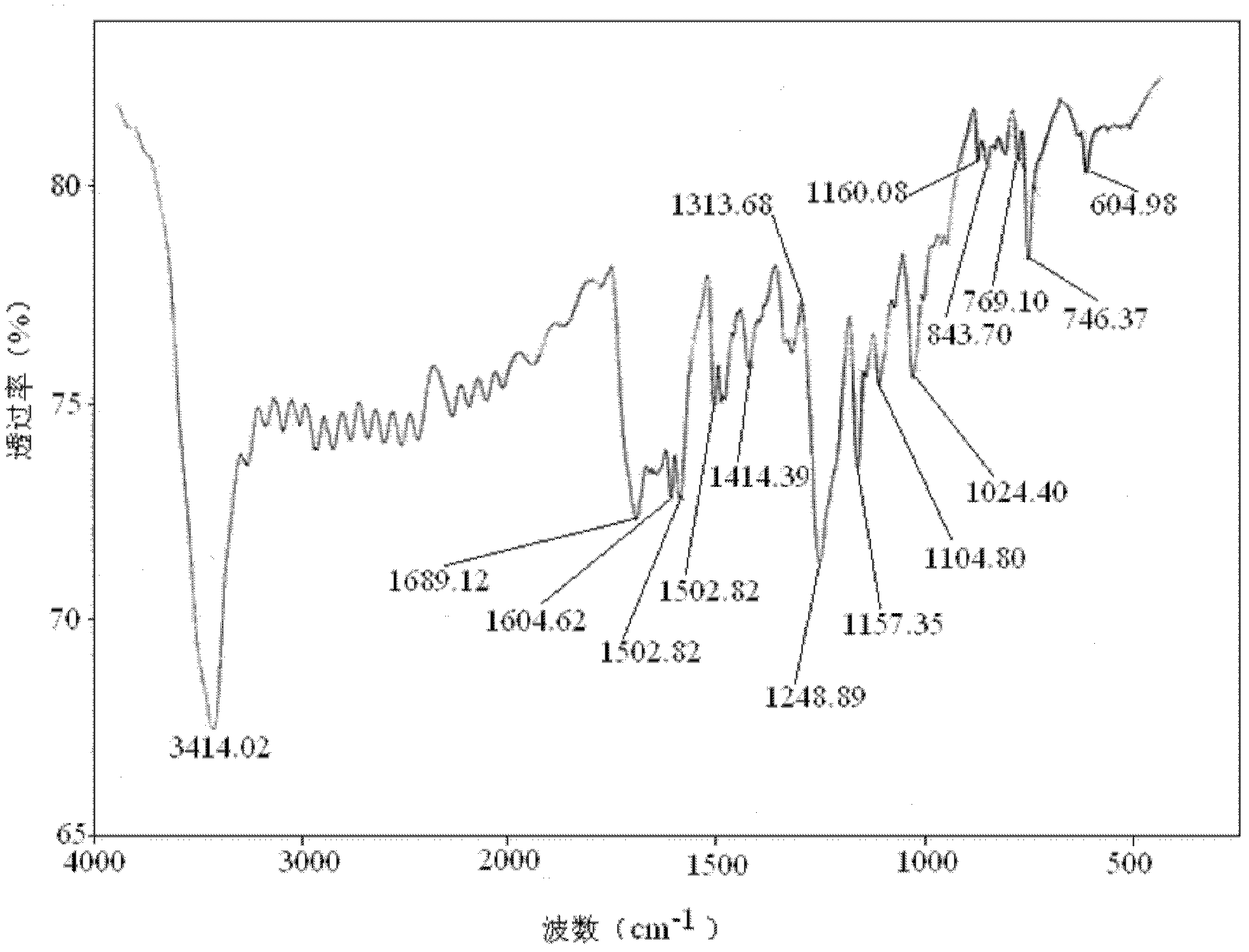
[0012] The α-tetrakis (4-carboxyphenoxy) phthalocyanine of the present embodiment has a symmetrical structure, and forms a double-layer assembly structure when self-assembling on the graphite surface. Not only there are large phthalocyanine π-conjugated bonds in the phthalocyanine ring, And there are π-conjugated bonds between the two layers of molecules, which increases the conjugation and has good chemical and thermal stability. α-Tetrakis(4-carboxyphenoxy)phthalocyanine has good solubility and film-forming properties in organic solvents.
specific Embodiment approach 2
[0013] Specific embodiment two: the preparation method of the α-tetra(4-carboxyphenoxy) phthalocyanine of the present embodiment is carried out in the following steps:
[0014] 1. The molar ratio of 3-(4-methoxyphenoxy)phthalonitrile to 1,8-diazabicyclo[5,4,0]undecene-7 is 1:1 ~2,3-(4-methoxyphenoxy)phthalonitrile and n-amyl alcohol in a molar ratio of 1:40~50 Weigh 3-(4-carboxyphenoxy)phthalonitrile Add formonitrile, 1,8-diazabicyclo[5,4,0]undecene-7 and n-amyl alcohol into a reactor with a reflux device, stir and heat to reflux under nitrogen protection, and keep Reflux for 24h to 36h; 2. Remove n-pentanol by distillation under reduced pressure. After cooling to normal temperature, add methanol, stir evenly, let stand for 12h to 24h, then filter, and wash the obtained solid phase with methanol to obtain α-tetra(4- Carbomethoxyphenoxy) phthalocyanine crude product; three, the α-tetrakis (4-methoxyphenoxy) phthalocyanine crude product that step 2 obtains is purified, obtains ...
specific Embodiment approach 3
[0018] Specific embodiment three: the difference between this embodiment and specific embodiment two is that in step one, 3-(4-methoxyphenoxy)phthalonitrile and 1,8-diazabicyclo[5,4 , 0] The molar ratio of undecene-7 is 1:1.1~1.8, and the molar ratio of 3-(4-methoxyphenoxy)phthalonitrile to n-pentanol is 1:41~49. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com