A kind of preparation method of rivastigmine
A synthesis method and technology of protecting groups, which are applied in the field of asymmetric synthesis of rivastigmine, can solve the problems of unrecoverable chiral inducer, poor atom economy, large amount of waste, etc., and achieve environmental friendliness, low cost and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the synthesis of (S)-1-m-methoxyphenylethylamine
[0030] (1) Synthesis of N-o-methoxyphenyl m-methoxyacetophenone imine
[0031] Under normal pressure and nitrogen protection, m-methoxyacetophenone (1.50 g, 10.0 mmol) and o-methoxyphenylamine (1.23 g, 10.0 mmol) were dissolved in toluene, and stirred at 0° C. for about 5 h. After the completion of the reaction as monitored by TLC, filter, wash with toluene (3×30 mL), and evaporate the solvent under reduced pressure to obtain 2.31 g of N-o-methoxyphenyl m-methoxyacetophenone imine, which is a light yellow solid with a yield of 90%. .
[0032](2) Synthesis of (S)-N-o-methoxyphenyl m-methoxyphenylethylamine
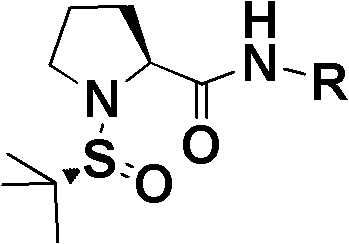
[0033] Under normal pressure and nitrogen protection, N-tert-butylsulfinylproline-2,6-diisopropylphenylamide catalyst (344mg, 0.91mmol) and N-o-methoxyphenyl m-methoxy Acetophenoneimine (2.31g, 9.06mmol) was mixed in toluene (20mL), cooled to 0°C, trichlorosilane (1.8mL, 18.1mmol) was added slowly, a...
Embodiment 2
[0036] Embodiment 2: the synthesis of (S)-1-m-methoxyphenylethylamine
[0037] (1) Synthesis of N-methoxyphenyl m-methoxyacetophenone imine
[0038] Under normal pressure and nitrogen protection, m-methoxyacetophenone (1.50 g, 10.0 mmol) and m-methoxyphenylamine (1.23 g, 10.0 mmol) were dissolved in toluene, and stirred at 0° C. for about 12 h. The post-treatment was the same as in Example 1 to obtain 2.34 g of N-m-methoxyphenyl-m-methoxyacetophenone imine as a pale yellow solid with a yield of 91%.
[0039] (2) Synthesis of (S)-N-m-methoxyphenyl-m-methoxyphenylethylamine
[0040] Under normal pressure and nitrogen protection, N-tert-butylsulfinyl proline tert-butyl amide catalyst (226mg, 0.86mmol) and N-methoxyphenyl m-methoxyacetophenone imine (2.34g , 9.18mmol) were mixed in carbon tetrachloride (20mL), cooled to -5°C, trichlorosilane (1.61mL, 16.1mmol) was added slowly, and reacted at -5°C for 10h. The specific operation steps were the same as in Example 1 to obtain 2.1...
Embodiment 3
[0043] Embodiment 3: the synthesis of (S)-1-m-methoxyphenylethylamine
[0044] (1) Synthesis of (S)-N-p-methoxyphenyl m-methoxyacetophenone imine
[0045] Under normal pressure and nitrogen protection, m-methoxyacetophenone (1.50 g, 10.0 mmol) and p-methoxyphenylamine (1.23 g, 10.0 mmol) were dissolved in dichloromethane, and stirred at 0° C. for about 5 h. The post-treatment was the same as in Example 1 to obtain 2.37 g of N-p-methoxyphenyl m-methoxyacetophenone imine as a light yellow solid with a yield of 92%.
[0046] (2) Synthesis of (S)-N-p-methoxyphenyl m-methoxyphenylethylamine
[0047] Under normal pressure and nitrogen protection, N-tert-butylsulfinylproline benzylamide catalyst (215mg, 0.698mmol) and N-p-methoxyphenyl m-methoxyacetophenone imine (2.37g, 9.3mmol) was mixed in dichloromethane (20mL), cooled to -10°C, trichlorosilane (1.40mL, 13.95mmol) was added slowly, and reacted at -10°C for 10h. The specific operation steps were the same as in Example 1 to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com