Preparation method of vanadium-nitrogen co-doped tio2 photocatalyst
A photocatalyst and co-doping technology, which is applied in the field of photocatalytic materials, can solve the problems of poor photocatalytic activity and unstable catalytic performance of photocatalysts, and achieve the effects of less TiO2 doping, excellent degradation performance, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
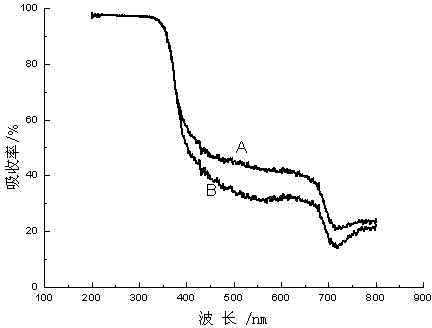
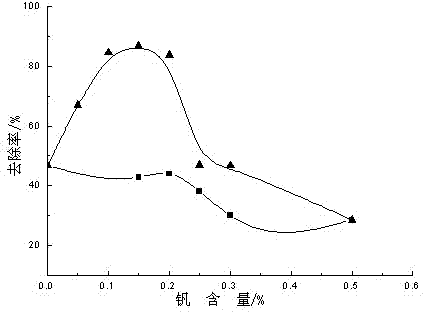
Image
Examples
Embodiment 1
[0028] Embodiment 1, accurately weigh 0.0018g ammonium metavanadate in a 50ml flask, add 5ml absolute ethanol, 0.3ml diethanolamine, 7ml butyl titanate, and add 0.1ml acetic acid, magnetically stir for 3h, make metavanadate Dissolve ammonium evenly; slowly drop into the mixture of 100ml of water and 15ml of acetic acid under ice-water bath and strong stirring, continue to stir for 1h after the dropwise addition, and put the reaction solution in a water bath at 328K for 24h, and then dry it at 373K. The final solid is ground to below 100 mesh, calcined at 723K for 3 hours, and the calcined powder is ground to below 100 mesh for later use to obtain vanadium-nitrogen co-doped TiO 2 . The resulting vanadium-nitrogen co-doped TiO 2 The degradation rate of phenol was 82.12%.
Embodiment 2
[0030] Accurately weigh 0.0060g of vanadium tetrachloride into a 50ml flask, add 12ml of absolute ethanol, 0.2ml of diethanolamine, 5ml of butyl titanate, and add 0.1ml of acetic acid, and stir magnetically for 3 hours to evenly dissolve vanadium tetrachloride; Under ice-water bath and strong stirring, slowly drop into the mixed solution of 90ml water and 5ml acetic acid, continue to stir for 3h, the reaction solution is in a water bath at 338K for 15h, and then dried at 373K, and the dried solid is ground to 100 mesh Next, calcined at 623K for 3 hours, and ground the calcined powder to below 100 mesh to obtain vanadium-nitrogen co-doped TiO 2 , the resulting vanadium-nitrogen co-doped TiO 2 The degradation rate of phenol was 74.10%.
Embodiment 3
[0032]Accurately weigh 0.0125g of vanadium pentachloride in a 50ml flask, add 20ml of absolute ethanol, 0.5ml of diethanolamine, 7ml of butyl titanate, and add 0.1ml of sulfuric acid with a concentration of 0.1mol / L, and magnetically stir for 1h to make Vanadium pentachloride is uniformly dissolved in the solution, and the above mixed solution is slowly dripped into the mixed solution of 125ml water and 15ml acetic acid under ice-water bath and strong stirring, after the dropwise addition, continue to stir for 2h, and the stirred reaction solution is Water bath at 318K for 12 hours, then dry at 373K, grind the dried solid to below 100 mesh, calcined at 773K for 2 hours, grind the calcined powder to below 100 mesh for later use, and obtain vanadium-nitrogen co-doped TiO 2 . The resulting vanadium-nitrogen co-doped TiO 2 The degradation rate of phenol was 86.95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com