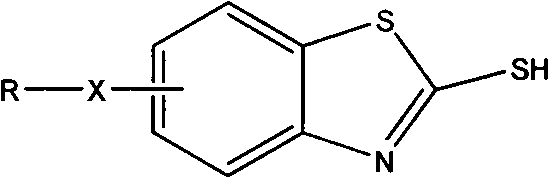
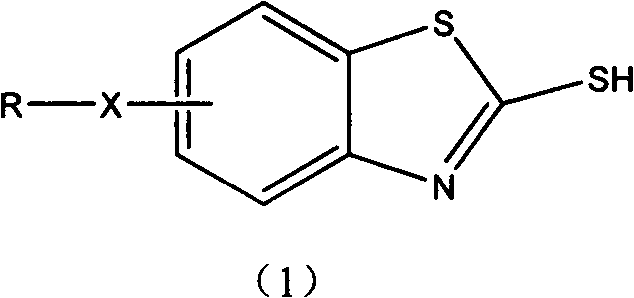
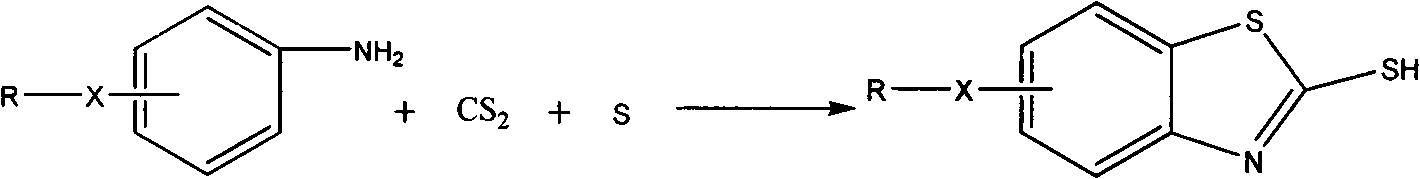Improved method for synthesizing 2-mercaptobenzothiazole derivative
A technology of mercaptobenzothiazole and synthesis method, which is applied in the field of synthesis of 2-mercaptobenzothiazole derivatives, can solve the problems of insufficient conversion rate and high production cost, and achieve simple raw materials, high reaction yield, and economical synthesis The effect of time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Add 59.6g of sulfur, 100g of carbon disulfide, and 123g of m-methoxyaniline into the reaction kettle, and stir at room temperature for 1h. The speed is 180-250r / min. Mix the reactants thoroughly. Seal the reactor well, fill the reactor with N 2 . The temperature was raised, and it took 40 minutes to raise the temperature to 200°C, and the reaction was stirred at this temperature for 3 to 5 hours. Cool after the reaction, dissolve the product with lye, and filter to remove insoluble by-products and resin impurities. The filtrate is acidified to obtain a product with a content of more than 99%. By optimizing the experimental conditions, the reaction yield can reach 96-100%.
Embodiment 2
[0014] Add 59.6g of sulfur, 100g of carbon disulfide, and 137g of m-ethoxyaniline into the reaction kettle, and stir for 1 hour at a speed of 180-250r / min. Mix the reactants thoroughly. Seal the reactor well, fill the reactor with N 2 . The temperature started to rise, and it took 40 minutes to raise the temperature to 208°C, and the reaction was stirred at this temperature for 4 to 5 hours. Cool after the reaction, dissolve the product with lye, and filter to remove insoluble by-products and resin impurities. The filtrate is acidified to obtain a product with a content of more than 99%. By optimizing the experimental conditions, the reaction yield can reach 96-100%.
Embodiment 3
[0016] Add 59.6g of sulfur, 100g of carbon disulfide, and 107g of o-toluidine into the reaction kettle, and stir for 1 hour at a speed of 180-250r / min. Mix the reactants thoroughly. Seal the reactor well, fill the reactor with N 2 . The temperature started to rise, and it took 70 minutes to raise the temperature to 240°C, and the reaction was stirred at this temperature for 3 to 4 hours. Cool after the reaction, dissolve the product with lye, and filter to remove insoluble by-products and resin impurities. The filtrate is acidified to obtain a product with a content of more than 99%. By optimizing the experimental conditions, the reaction yield can reach 94-100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com