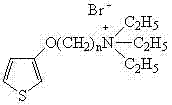
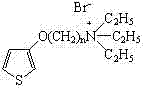
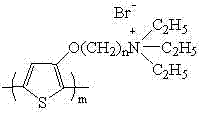
Water-soluble thiophene monomer and water-soluble polythiophene derivative as well as preparation methods of water-soluble thiophene monomer and water-soluble polythiophene derivative
A water-soluble polythiophene and water-soluble technology, applied in the field of organic polymer optoelectronic materials, can solve problems such as damage and affect the performance of organic light and electrical devices, and achieve the effect of water-solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1), the preparation of water-soluble thiophene monomer:
[0030] In the first step, at N 2 under protection, will N - Methylpyrrolidone and sodium metal were added to a three-necked flask, methanol was slowly added dropwise, and the reaction was stirred at room temperature for 3 h until the sodium metal disappeared. Residual methanol was distilled off under reduced pressure to obtain sodium methylate N - methylpyrrolidone solution;
[0031] In the second step, N 2 Under protection, add the above-mentioned sodium methylate in the three-necked flask N -Methylpyrrolidone solution, cuprous bromide catalyst and 3-bromothiophene, stirred and reacted for 8 h at 100°C, poured the solution into dichloromethane after the reaction, filtered to obtain a reddish-brown solution, in the reddish-brown solution Add saturated sodium chloride solution, extract with dichloromethane, extract the organic phase, dry over anhydrous sodium sulfate, and filter to remove sodium sulfate. Dic...
Embodiment 2
[0043] 1) Preparation of water-soluble thiophene derivative monomer:
[0044] In the first step, at N 2 under protection, will N- Methylpyrrolidone and sodium metal were added to a three-necked flask, methanol was slowly added dropwise, and the reaction was stirred at room temperature for 4 h until the sodium metal disappeared. Residual methanol was distilled off under reduced pressure to obtain sodium methylate N - methylpyrrolidone solution;
[0045] In the second step, N 2 Under protection, add the above-mentioned sodium methylate in the three-necked flask N -Methylpyrrolidone solution, cuprous bromide catalyst and 3-bromothiophene, stirred and reacted for 5 h at 105°C, poured the solution into dichloromethane after the reaction, filtered to obtain a reddish-brown solution, in the reddish-brown solution Add saturated sodium chloride solution, extract with dichloromethane, extract the organic phase, dry over anhydrous sodium sulfate, and filter to remove sodium sulfate....
Embodiment 3
[0057] 1) Preparation of water-soluble thiophene derivative monomer:
[0058] In the first step, at N 2 under protection, will N - Methylpyrrolidone and sodium metal were added to a three-necked flask, methanol was slowly added dropwise, and the reaction was stirred at room temperature for 3.5 h until the sodium metal disappeared. Residual methanol was distilled off under reduced pressure to obtain sodium methylate N - Methylpyrrolidone solution.
[0059] In the second step, N 2 Under protection, add the above-mentioned sodium methylate in the three-necked flask N -Methylpyrrolidone solution, cuprous bromide catalyst and 3-bromothiophene, stirred and reacted for 4 h at 110°C, poured the solution into dichloromethane after the reaction, filtered to obtain a reddish-brown solution, in the reddish-brown solution Add saturated sodium chloride solution, extract with dichloromethane, extract the organic phase, dry over anhydrous sodium sulfate, and filter to remove sodium sulfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com