High penetration compositions and their applications
A penetrating, compositional technology for the prevention, diagnosis and/or treatment of symptoms or diseases in humans, animals and plants, capable of addressing patient pain, needle sticks, infections, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
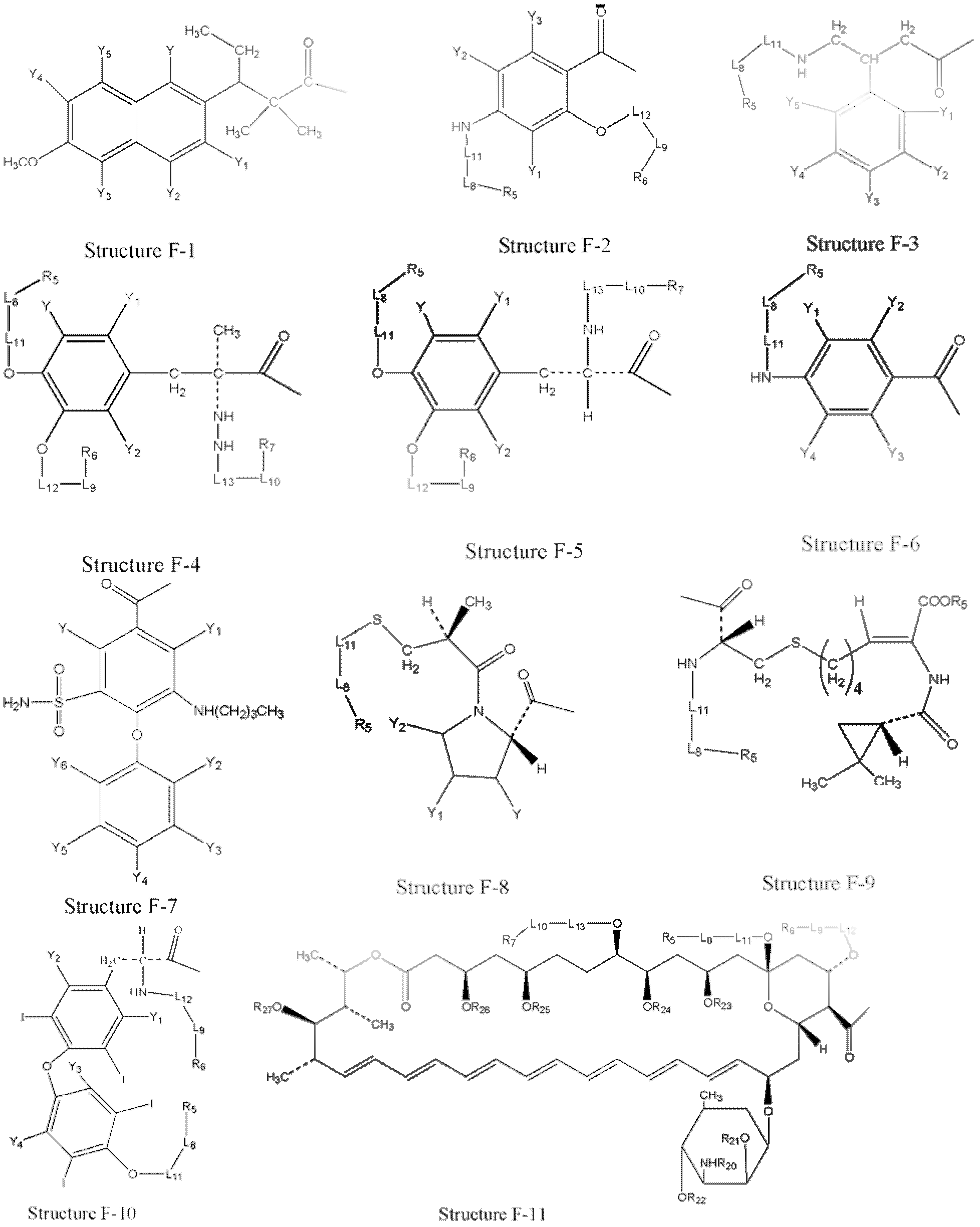
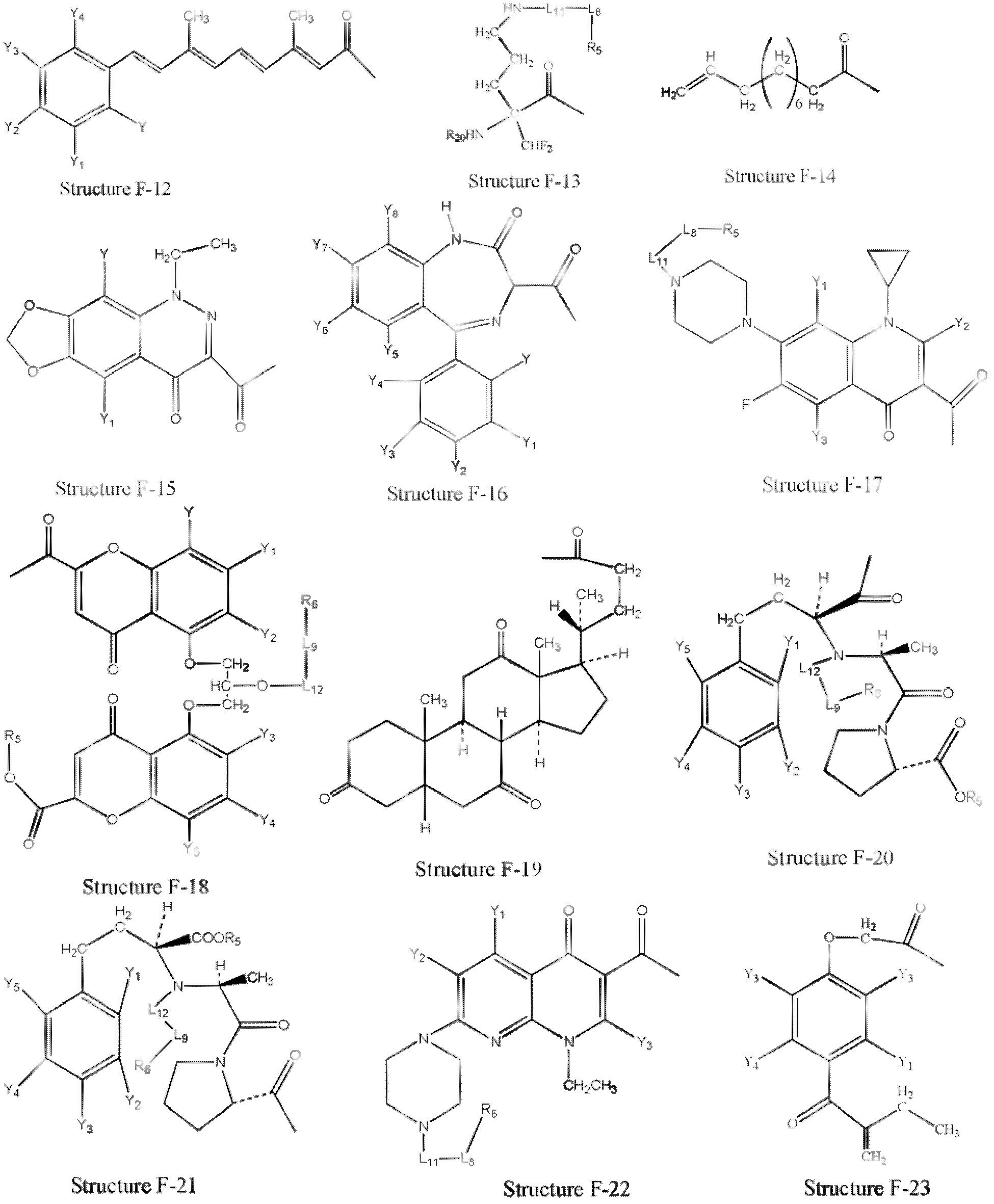
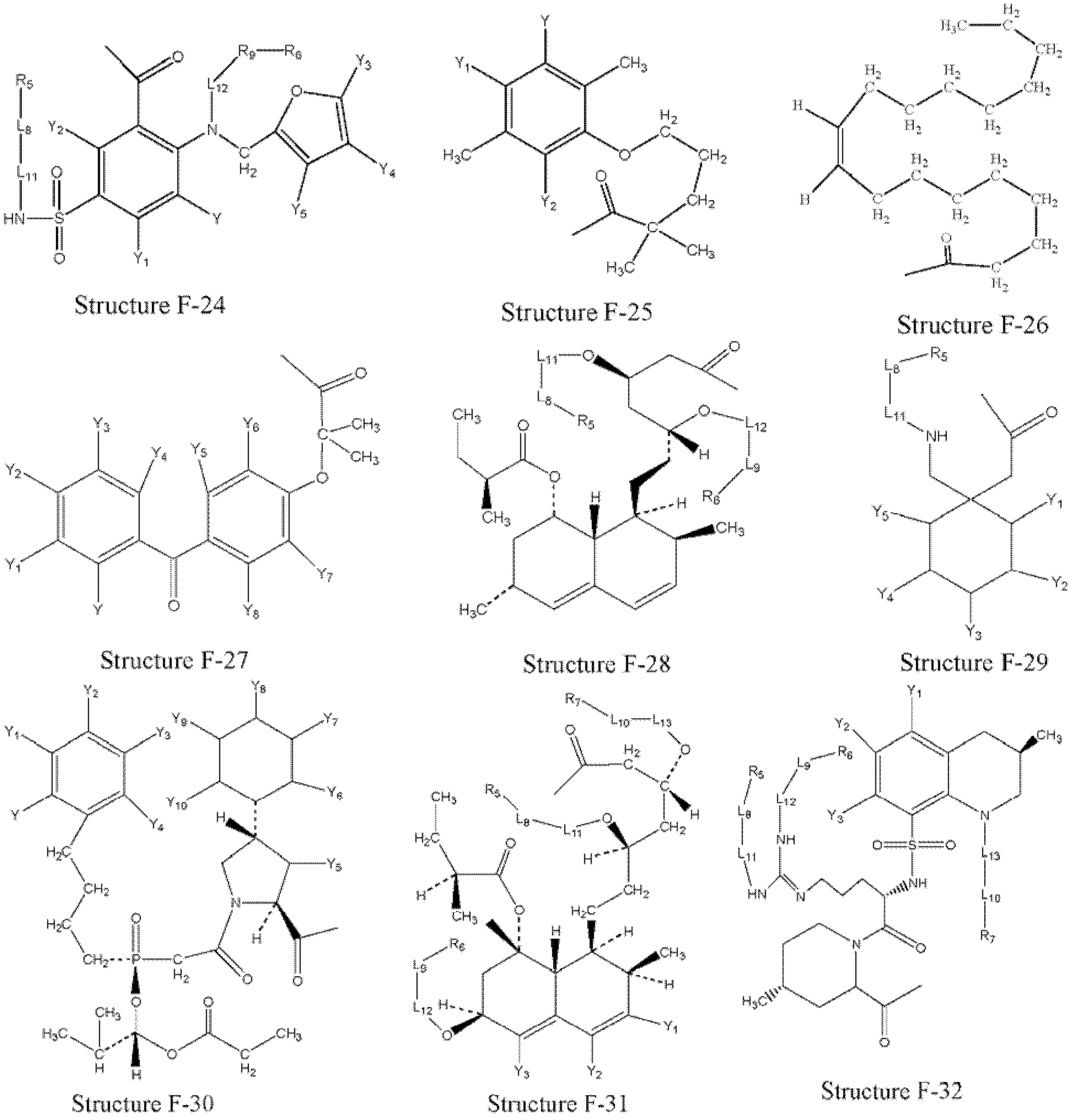
[0321] Example 1: Preparation of HPCs from parent drug
[0322] HPCs are prepared from parent drugs containing at least one carboxyl group.
[0323] In certain embodiments, structural formula F 1 The parent drug represented by -OH can be transformed into a HPC represented by structural formula L-1:
[0324] f 1 -L 2 -T
[0325] Structural formula L-1
[0326] Stereoisomers and pharmaceutically acceptable salts thereof are included, wherein T is defined to be consistent with T in paragraph 0076.
[0327] In certain embodiments, structural formula L-1(F 1 -L 2 -T) represented HPC by structural formula F 1 -W a The represented parent compound or its derivatives (such as acid halides, mixed anhydrides, etc. of the parent drug) are the same as those of the structural formula T-L in Fig. 2 The compound represented by -H is prepared by organic synthesis reaction, wherein: W a selected from OH, halogen, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy, and aryloxycarbon...
Embodiment 2
[0370] Example 2. HPCs capable of penetrating biological barriers
[0371] The rate of penetration of HPCs through human skin is measured in vitro using a modified Franz cell. The Franz cell has two chambers, a top sample chamber and a bottom receiving chamber. Human skin tissue (360-400 [mu]m thick) separating the apical and receptive compartments was obtained from the anterior and posterior regions of the thigh.
[0372] The test compounds are N-acetyl-3-(3,4-diacetoxy-phenyl-L-alanine diethylaminoethyl ester hydrochloride (A), diethylaminopropyl N-acetyl Base-D-3,5,3',5'-tetraiodothyronine hydrochloride (B), 1-piperidinyl-2[4-(4-chlorobenzoyl)phenoxy -2-methyl-propionate hydrochloride (C), 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic acid 3-piperidine methyl ester hydrochloride (D ), (S)-3-(Benzamidomethyl)-5-methylhexanoic acid diethylaminoethyl ester hydrochloride (E), N-acetyl-3-(3,4-diacetyl Oxy-phenyl-L-alanine (F) sodium salt, N-acetyl-D-3,5,3',5'-tetraiodothyron...
Embodiment 3
[0378] Example 3: After transdermal administration of the HPC, the distribution of the parent drug and related compounds in the body.
[0379] The HPC of ibuprofen in this example is diethylaminoethyl 2-(p-isobutylphenyl)propionate citrate. The HPC is rapidly converted to its parent drug in vivo, so the concentration detected is that of the parent drug and its related compounds.
[0380] 3.1 After the HPC transdermal administration of ibuprofen, detect the distribution of parent drug and related compounds in rats
[0381] The ibuprofen HPC (10% aqueous solution) of 0.3mmol / kg is coated on the back (10cm 2 ). Table 3 shows the distribution of ibuprofen and diethylaminoethyl 2-(p-isobutylphenyl) propionate hydrochloride (bulomide) in rat organs 2 hours after administration.
[0382] Table 3.1 Distribution of ibuprofen and ibuproamine in rat organs
[0383]
[0384] 3.2 The HPC of ibuprofen can disperse the mother drug ibuprofen and its related compounds to various organs ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com