A compound of N- nitro-2,4,6-trifluoroaniline, synthesis method and application thereof
A technology of trifluoroaniline and compound is applied in the field of compound N-nitro-2, and achieves the effects of simple process, few steps and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
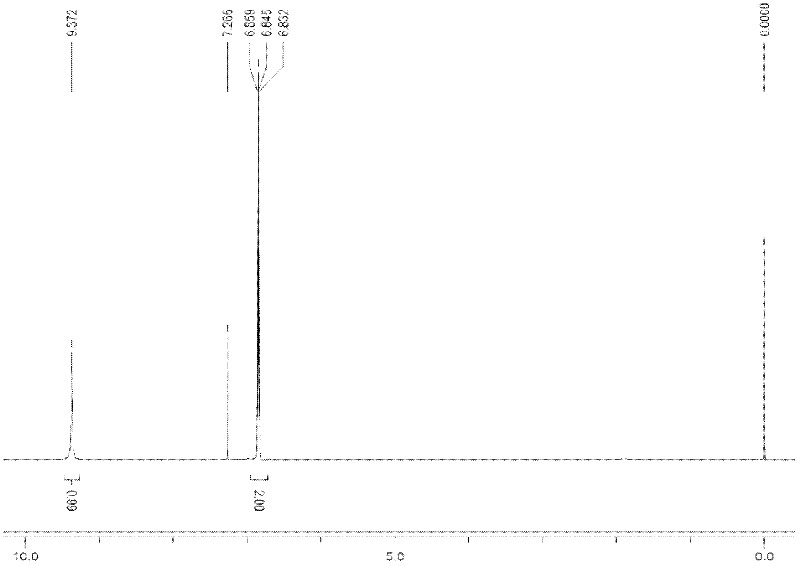
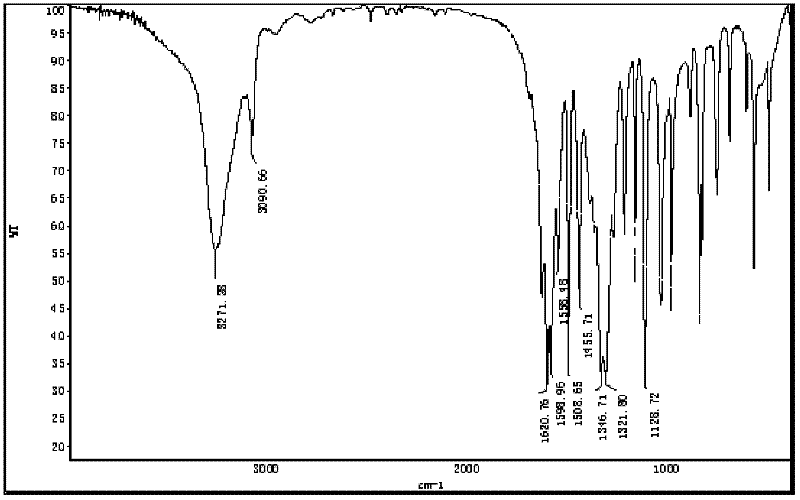
Image
Examples
Embodiment 1
[0027] The synthetic method of compound N-nitro-2,4,6-trifluoroaniline comprises the following steps:
[0028] (1) Add 40ml toluene, 11.77g (0.08mol) 2,4,6-trifluoroaniline (purchased from Changzhou Yetai Fine Chemical Industry Co., Ltd. Research Institute), water bath temperature control 20 ℃, magnetic stirring, slowly dropwise add a mixture of 6ml of fuming nitric acid with a concentration of 98% and 10ml of toluene, the dropwise addition is completed in 20 minutes, continue to stir for 30 minutes, and stop the reaction. Suction filtration under reduced pressure, washing with 10ml of toluene to obtain a white powder solid, namely 2,4,6-trifluoroaniline nitrate, vacuum drying, the yield was 96%;
[0029] (2) Add 100ml toluene, 10.5g (0.05mol) 2,4,6-trifluoroaniline nitrate in a 250ml three-necked flask equipped with magnetic stirring, thermometer, constant pressure dropping funnel and reflux condenser, and control the temperature at 25 -30°C, slowly add a mixture of 8ml acet...
Embodiment 2
[0037] Test of Plant Growth Regulatory Activity of Compound N-nitro-2,4,6-trifluoroaniline
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com