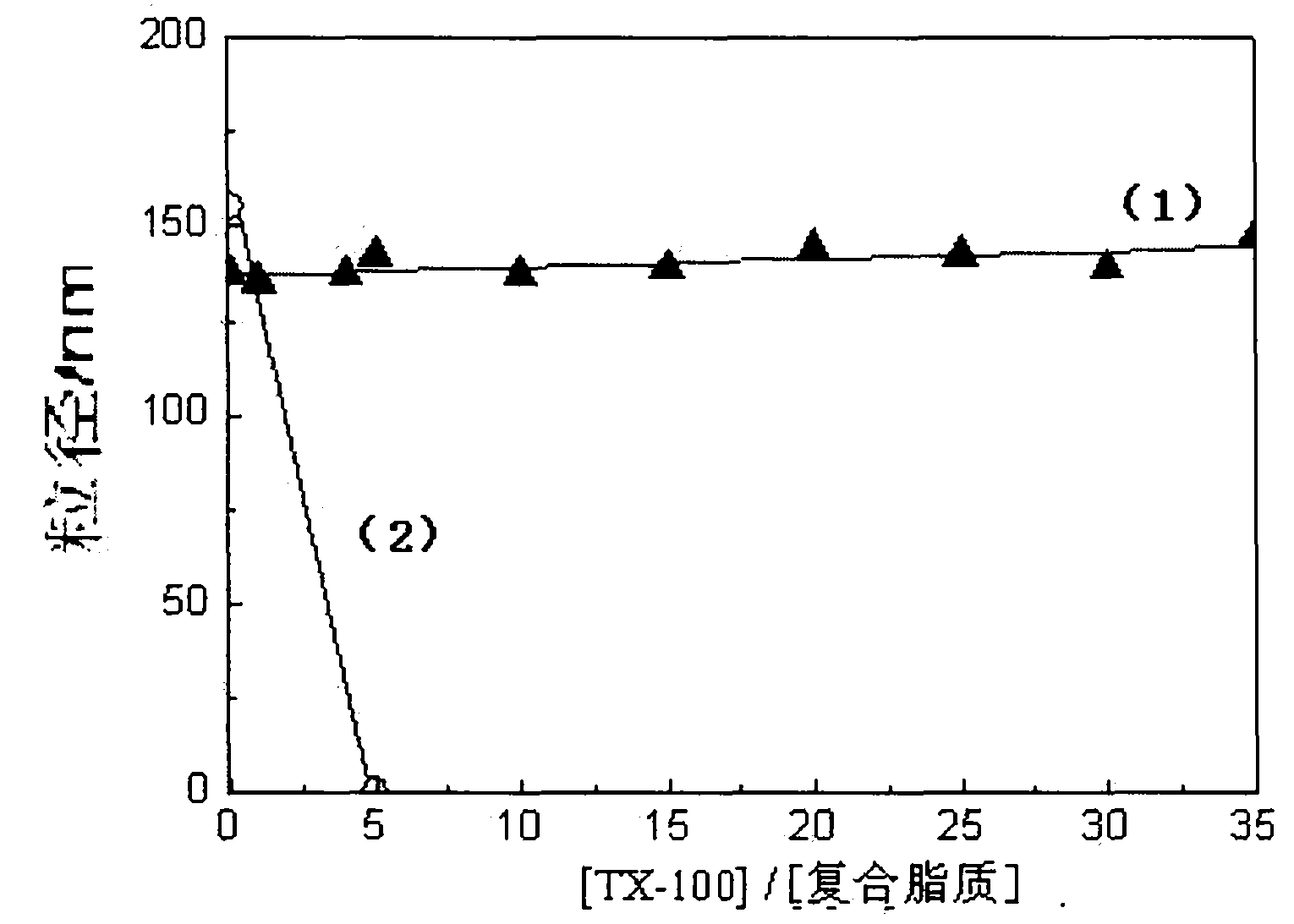
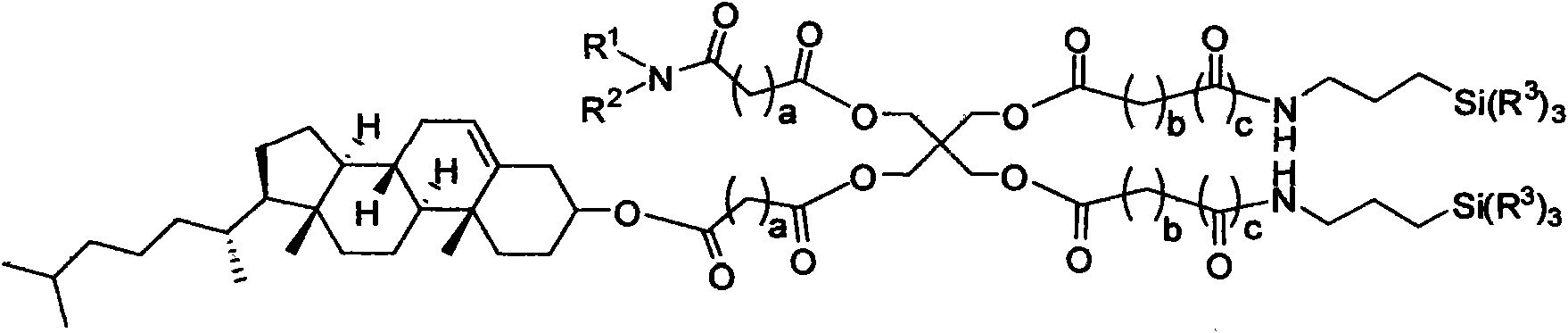
Composite lipid comprising cholesterol group and intermediate, preparation method as well as application thereof
A cholesterol group and compound lipid technology, which is applied in the field of biomedical materials, can solve the problems of internal drug leakage, decreased liposome stability, increased mechanical properties, etc., and achieves strong operability, cheap raw materials, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] After mixing 1 mmol of compound 1 and 2 mmol of compound 2, dissolved in 40 mL of dimethylformamide, heated to complete dissolution, then added 2 mmol of DCC and 1 mmol of DMAP in turn, stirred at 55 ° C for 20 hours, reduced The solvent was evaporated to dryness under pressure, and the obtained crude product was separated and purified by column chromatography to obtain compound 3 in a yield of 80%.
[0027] C 79 H 133 NO 9
[0028] 1 H NMR (CDCl 3 , 400MHz)δ: 0.85~2.92(m, 15H, CH 3 ), 0.99(s, 3H, CH 3), 1.09~1.60(m, 70H), 1.81~1.85(m, 2H), 2.31(d, J=7.6Hz, 2H, CH 2 ), 2.60~2.68(m, 8H, NCOCH 2 CH 2 CO), 3.22~3.28(m, 4H, NCH 2 ), 3.87~4.51(m, 8H, OCH 2 ), 4.44~4.45(m, 1H, COOCHCH 2 ), 5.35(m, 1H, C=CCH) 5.44(s, 1H, PhCHOCH 2 ), 7.34~7.48(m, 5H, Ph-H). Theoretical value of mass spectrum: 1240.9, experimental value [M] + : 1241.5, [M+Na] + : 1263.6.
Embodiment 2
[0030] The compound 3 of 2mmol (2.48g) was dissolved in the mixed solvent of methanol and tetrahydrofuran with a volume ratio of 1:3, added to a 250mL reactor, then added 1.24g of palladium hydroxide / carbon, and vigorously stirred at 50°C For 48 hours, the solvent was evaporated to dryness under reduced pressure, and the obtained crude product was separated and purified by column chromatography to obtain compound 4 in a yield of 52%.
[0031] C 72 H 129 NO 9
[0032] 1 H NMR (CDCl 3 , 400MHz)δ: 0.85~0.92(m, 15H, CH 3 ), 1.01(s, 3H, CH 3 ), 1.08~1.60(m, 70H), 1.81~1.85(m, 2H), 2.31(d, J=7.6Hz, 2H, CH 2 ), 2.62~2.68(m, 8H, COCH 2 CH 2 CO), 3.19~3.28(m, 4H, NCH 2 ), 3.58(s, 4H, HOOCH 2 ), 4.16~4.19(m, 4H, COOCH 2 ), 4.44~4.45(m, 1H, COOCHCH 2 ), 5.36 (d, J=4Hz, 1H, C=CH). Theoretical value of mass spectrum: 1152.80, experimental value [M] + : 1153.5, [M+Na] + : 1175.5.
Embodiment 3
[0034] Under nitrogen protection, 1 mmol of compound 4 was dissolved in 40 mL of dichloromethane, 2.5 mmol of compound 5 and 0.4 mmol of catalyst dibutyltin dilaurate were added in sequence, and the mixture was stirred at 55° C. for 48 hours. The crude product was separated and purified by column chromatography to obtain compound 6 in a yield of 53.2%.
[0035] C 92 H 171 N 3 O 17 Si 2
[0036] 1 H NMR (CDCl 3 , 400MHz)δ: 0.61(t, J=8.4Hz, 4H, SiCH 2 CH 2 CH 2 NH), 0.67 (s, 3H, CH 3 ), 0.86~1.10(m, 18H, CH 3 ), 1.10~1.35(m, 89H), 1.45~1.62(m, 12H), 1.81~2.30(m, 5H), 2.60~2.65(m, 8H, COCH 2 CH 2 CO), 3.20~3.36(m, 8H, SiCH 2 CH 2 CH 2 NH and CH 3 (CH 2 ) 13 CH 2 CH 2 N), 3.68~3.83(m, 12H, SiOCH 2 CH 3 ), 4.00~4.13(m, 8H, COOCH 2 C), 4.61~4.64 (m, 1H, COOCHCH 2 ), 5.38 (d, J=4Hz, 1H, C=CH). MS theoretical value: 1647.52, experimental value [M] + : 1648.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com