Acenaphthene heterocyclic compounds and application thereof
A compound and heterocyclic technology, applied in the field of acenaphthoheterocyclic Bcl-2 family protein inhibitors, can solve the problems of insufficient BH3 similarity, complex apoptosis signaling pathway, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
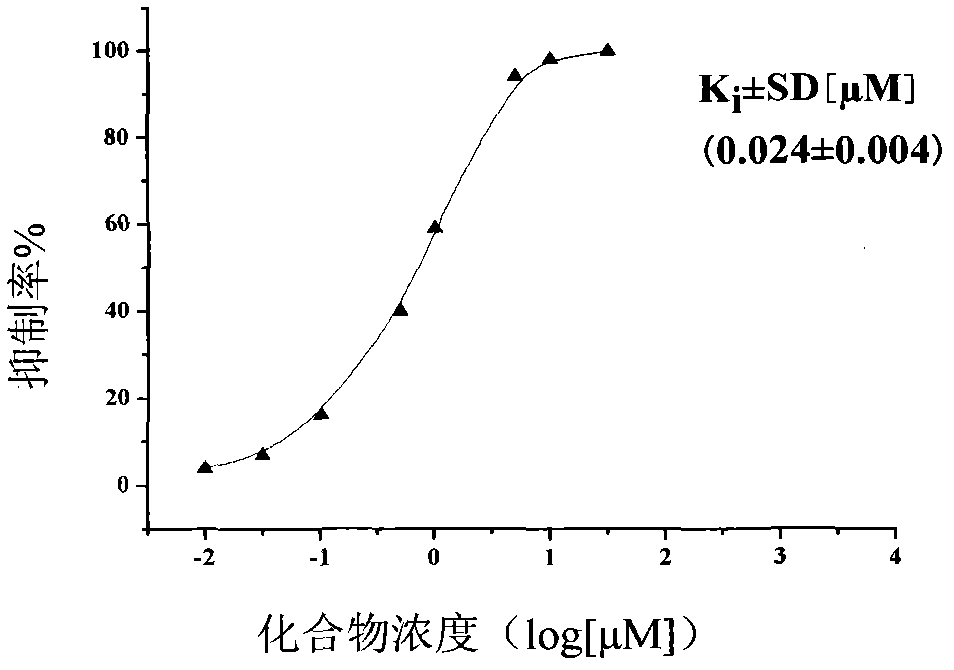
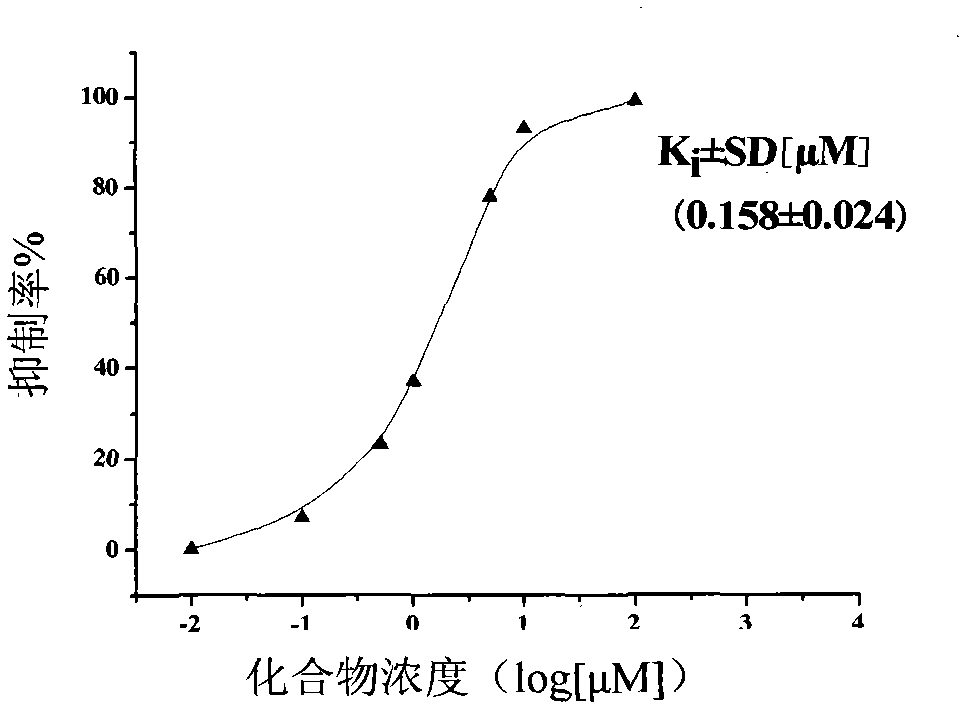
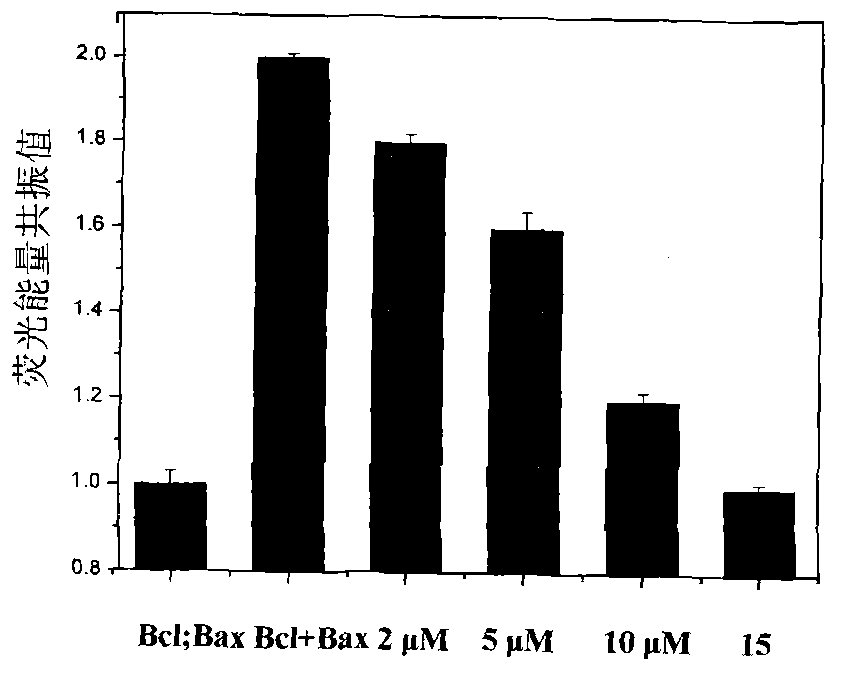
Image
Examples
Embodiment 1
[0053] Example 1: 3-(4-sec-butylphenoxy)-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (compound 1) and 4-(4-sec-butyl Synthesis and Characterization of Phenoxy)phenoxy-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (Compound 2)
[0054]
[0055] Weigh 0.99g of 5-(4-sec-butylphenoxy)acenaphthenequinone, dissolve 0.33g of malononitrile in dichloromethane, add to the silica gel column, rinse quickly, spin dry after passing through the column to obtain a red solid. Weighed 1.07g, yield 94%. Get 0.77g gained red solid, add 0.05g K 2 CO 3 , 20ml of acetonitrile was heated to reflux for 3 hours, and the reaction solution was spin-dried after the reaction was completed. Chromatographic column separation (CH 2 Cl 2 : petroleum ether=1:1) to obtain two isomers.
[0056] Compound 1: M.p.219-220°C. 1 H NMR (400M, CDCl 3 ): δ8.92(d, J=8.0Hz, 1H), 8.65(d, J=8.8Hz, 1H), 8.46(d, J=8.0Hz, 1H), 7.87(t, J=8.0Hz, 1H ), 7.33(d, J=8.4Hz, 2H), 7.14(d, J=8.4Hz, 2H), 7.04(d, J=8.0Hz,...
Embodiment 2
[0058] Example 2: 3-(4-isobutylphenoxy)-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (compound 3) and 4-(4-isobutyl Synthesis and Characterization of Phenoxy)phenoxy-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (Compound 4)
[0059]
[0060] Weigh 0.99g of 5-(4-isobutylphenoxy)acenaphthenequinone, dissolve 0.33g of malononitrile in dichloromethane, add to a silica gel column, rinse quickly, spin dry after passing through the column to obtain a red solid. Weighed 1.07g, yield 94%. Get 0.77g gained red solid, add 0.05g K 2 CO 3 , 20ml of acetonitrile was heated to reflux for 3 hours, and the reaction solution was spin-dried after the reaction was completed. Chromatographic column separation (CH 2 Cl 2 : petroleum ether=1:1) to obtain two isomers.
[0061] Compound 3: M.p.214-215°C. 1 H NMR (400M, CDCl 3 ): δ8.78(d, J=7.6Hz, 1H), 8.60(d, J=8.0Hz, 1H), 8.43(d, J=7.6Hz, 1H), 7.67(t, J=8.0Hz, 1H ), 7.29(d, J=8.0Hz, 2H), 7.12(d, J=8.0Hz, 2H), 6.95(d, J=8.0Hz, 1H),...
Embodiment 3
[0063] Example 3: Synthesis and characterization of 3-(4-isopropylphenoxy)-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (compound 5)
[0064]
[0065] 1g of 8-oxo-8H acenaphtho[1,2-b]pyrrole-9-carbonitrile and 0.54g of p-isopropylphenol were added to 50 ml of acetonitrile, refluxed for 3 hours, the solvent was distilled off, and compound 5 was obtained by column chromatography. rate 30%.
[0066] Compound 5: M.p.272-274°C; 1 H NMR (400M, CDCl 3 ): δ8.92(d, J=8.0Hz, 1H), 8.25(d, J=8.8Hz, 2H), 8.44(d, J=8.0Hz, 1H), 7.86(t, J=8.0Hz, 1H ), 7.38(d, J=8.4Hz, 2H), 7.14(d, J=8.4Hz, 2H), 7.04(d, J=8.8Hz, 1H), 3.01(m, 1H), 1.32(d, J =8.0Hz, 6H); TOF MS EI + (m / z): C 24 h 16 N 2 o 2 , calculated value: 364.1212, measured value: 364.1215.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com