Detection method of methylprednisolone sodium succinate freeze-dried powder injection related substance
A technology of methylprednisolone sodium succinate and freeze-dried powder injection, which is applied in the detection field of methylprednisolone sodium succinate freeze-dried powder injection, and can solve the problems of inability to effectively separate impurities, unsuitable detection substances, and unpublished quality detection methods, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
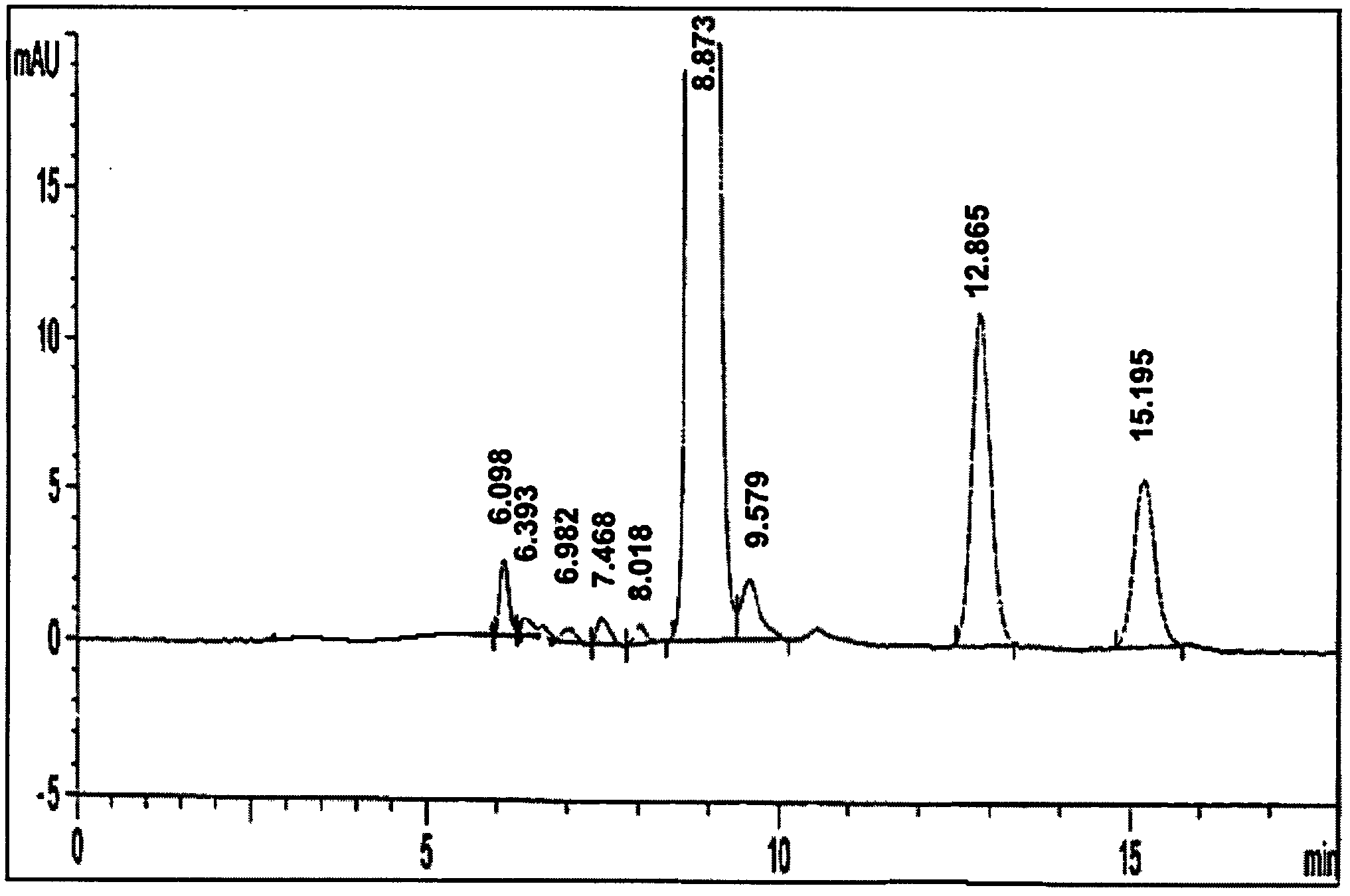
[0033] Existing HPLC conditions for detecting content and free methylprednisolone content are used to detect related substances, and normalization method is used without internal standard substance.
[0034] The detection method is as follows
[0035] Chromatographic conditions Chlorobutane-water saturated chlorobutane-tetrahydrofuran-methanol-glacial acetic acid (475:475:70:35:30) is the mobile phase; the detection wavelength is 254nm.
[0036] Preparation of Determination Method Reference Substance Solution Accurately weigh an appropriate amount of methylprednisolone succinate (about 15.84mg), put it in a 50ml measuring bottle, add 5ml of tetrahydrofuran, add 3% glacial acetic acid chloroform to dissolve and dilute to the scale, shake well, and accurately measure Take 1ml, put it in a 100ml measuring bottle, add 3% glacial acetic acid chloroform solution to dilute to the mark, shake well, and you get it.
[0037] Preparation of the test solution Precisely weigh an appropria...
Embodiment 2
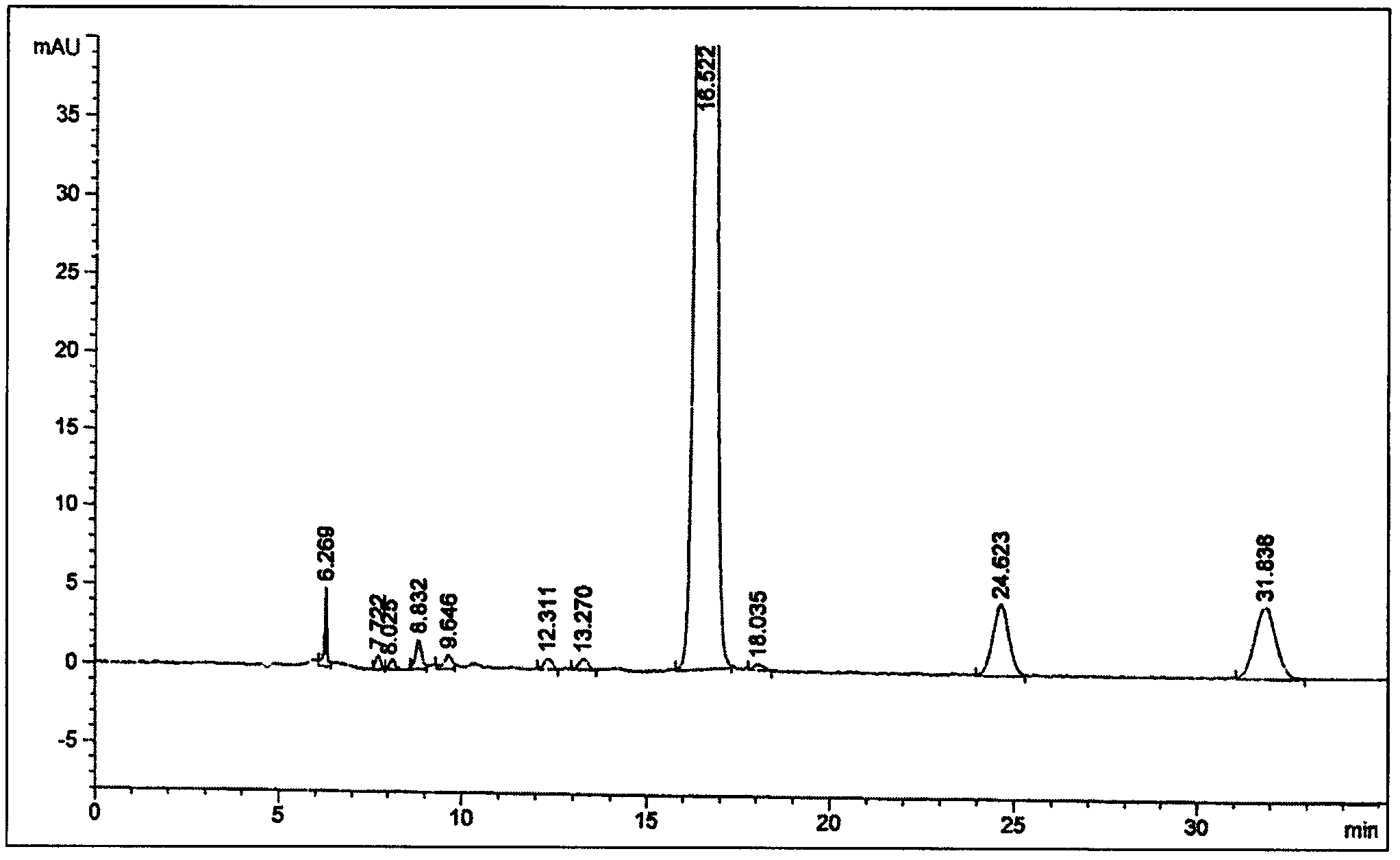
[0041] Adopt the method provided by the invention to carry out related substance content detection
[0042] The content of relevant substances adopts the following methods:
[0043] Chromatographic conditions The mobile phase is n-chlorobutane-water saturated n-chlorobutane-tetrahydrofuran-methanol-glacial acetic acid with a volume ratio of 130:130:14:7:6; the detection wavelength is 254nm.
[0044] Preparation of reference solution Accurately weigh an appropriate amount of methylprednisolone succinate (about 15.84mg), put it in a 50ml measuring bottle, add 5ml of tetrahydrofuran, add 3% glacial acetic acid chloroform to dissolve and dilute to the mark, shake well, and accurately measure 1ml , put it in a 100ml measuring bottle, add 3% glacial acetic acid chloroform solution to dilute to the mark, shake well, and you get it.
[0045] Preparation of the test solution Precisely weigh an appropriate amount of the test (approximately equivalent to 0.25g of methylprednisolone), pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com