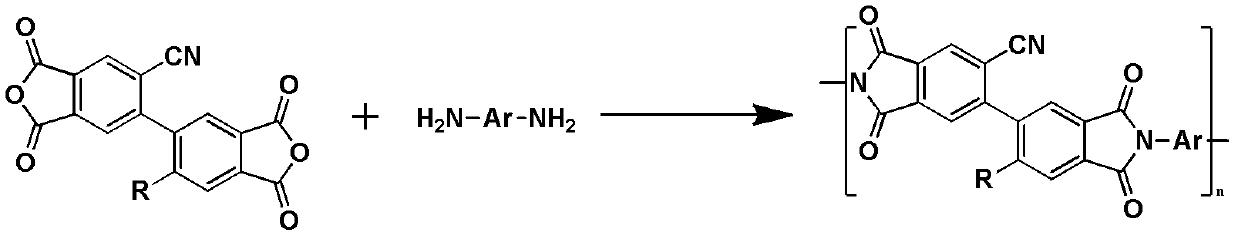
Modified biphenyl type dianhydride intermediate containing cyano side chain and synthesis method and application thereof
A technology of biphenyl tetraacid dianhydride and biphenyl type, which is applied in the field of cyano side chain modified biphenyl type dianhydride intermediates and its synthesis, which can solve poor solubility, difficult material processing, high melting temperature, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
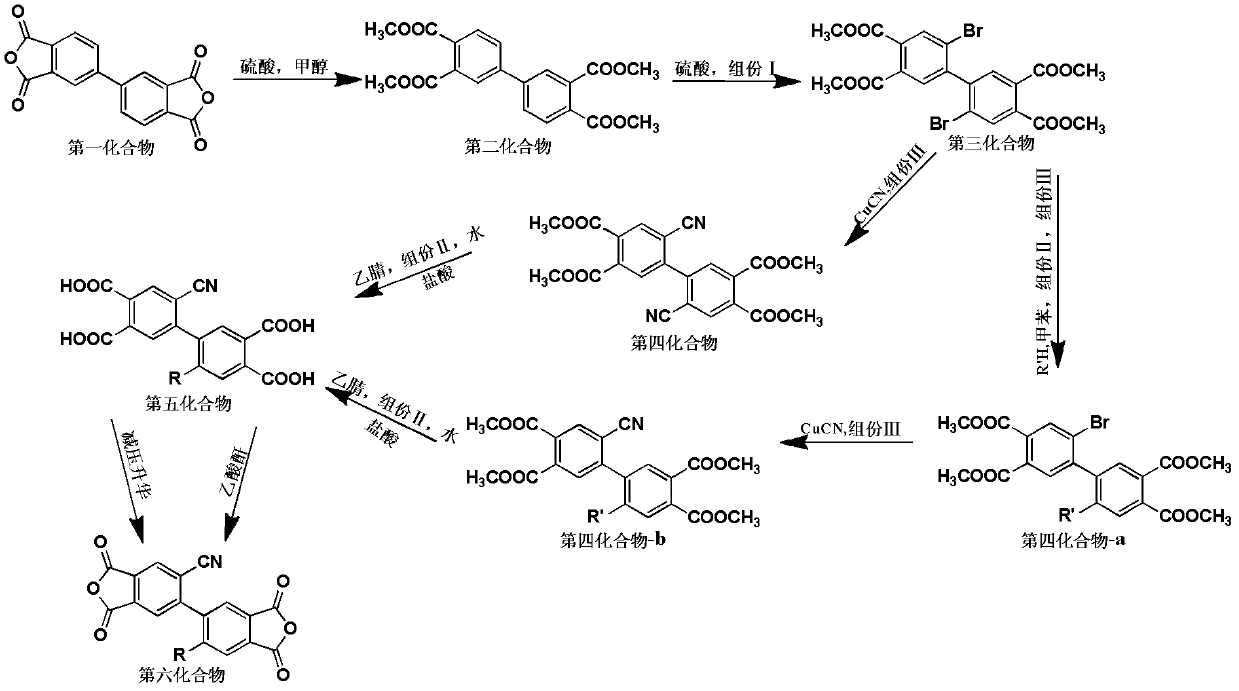
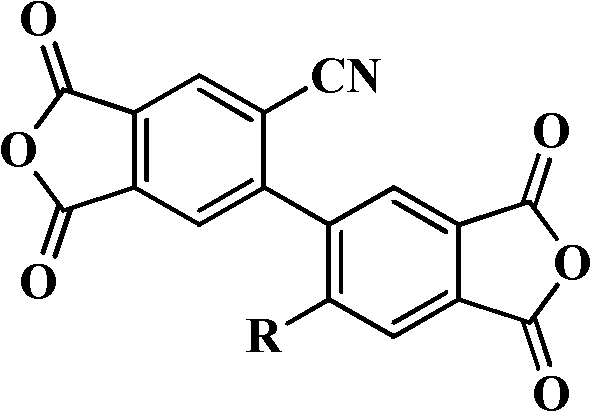
[0076] Embodiment 1: 2,2'-dicyano-4,4',5,5'-biphenyltetraacid dianhydride preparation (former example 2)
[0077] The processing steps of the present embodiment are as follows:
[0078] (1) raw material is 20.00g biphenyltetraic dianhydride and 440mL composite solvent, described composite solvent is formulated by 400mL methanol and 40mL vitriol oil; Add biphenyltetrapic dianhydride in the flask, then add described composite solvent , and then heated to 85°C under normal pressure to reflux for 16 hours. After the reflux time expired, stand still for 12 hours and filter. The obtained filter cake was washed with methanol and distilled water to remove sulfuric acid, and dried in an oven at 70°C for 12 hours to obtain White tetramethyl biphenyl tetracarboxylate 23.36g, yield 88.97%; 1 HNMR (400MHz, CDCl 3 -d 6 ): δ8.73 (s, 2H), 8.22 (d, 2H), 7.96 (d, 2H), 3.88 (s, 12H).
[0079] (2) raw material is 15.00g tetramethyl biphenyl tetracarboxylate, sulfuric acid and 16.22g sodium br...
Embodiment 2
[0084] Example 2: Preparation of 2-dicyano-2'-phenoxy-4,4',5,5'-biphenyltetraacid dianhydride
[0085] The processing steps of the present embodiment are as follows:
[0086] (1) raw material is 25.00g biphenyltetraic dianhydride and 394mL composite solvent, described composite solvent is formulated by 375mL methyl alcohol and 19mL vitriol oil; Add biphenyltetraic dianhydride in the flask, then add described composite solvent , and then heated to 80°C under normal pressure to reflux for 10 hours. After the reflux time expired, stand still for 10 hours and filter. The obtained filter cake was washed with methanol and distilled water to remove sulfuric acid, and dried in an oven at 70°C for 12 hours to obtain a white The yield of tetramethyl biphenyl tetracarboxylate is 87.44%; 1 HNMR (400MHz, CDCl 3 -d 6 ): δ8.73 (s, 2H), 8.22 (d, 2H), 7.96 (d, 2H), 3.88 (s, 12H).
[0087] (2) raw material is 15.00g tetramethyl biphenyl tetracarboxylate, sulfuric acid and 13.90g potassium b...
Embodiment 3
[0093] Example 3: Preparation of 2-dicyano-2'-p-tert-butylphenoxy-4,4',5,5'-biphenyltetraacid dianhydride
[0094] The processing steps of the present embodiment are as follows:
[0095] (1) raw material is 20.00g biphenyl tetraacid dianhydride and 575mL composite solvent, described composite solvent is formulated by 500mL methanol and 75mL vitriol oil; Add biphenyl tetraacid dianhydride in the flask, then add described composite solvent , and then heated to 95°C under normal pressure to reflux for 24 hours. After the reflux time expired, stand still for 12 hours and filter. The obtained filter cake was washed with methanol and distilled water to remove sulfuric acid, and dried in an oven at 70°C for 12 hours to obtain a white Tetramethyl biphenyl tetracarboxylate, yield 82.88%; 1 HNMR (400MHz, CDCl 3 -d 6 ): δ8.73 (s, 2H), 8.22 (d, 2H), 7.96 (d, 2H), 3.88 (s, 12H).
[0096] (2) raw material is 15.00g tetramethyl biphenyl tetracarboxylate, sulfuric acid and 14.28g potassiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com