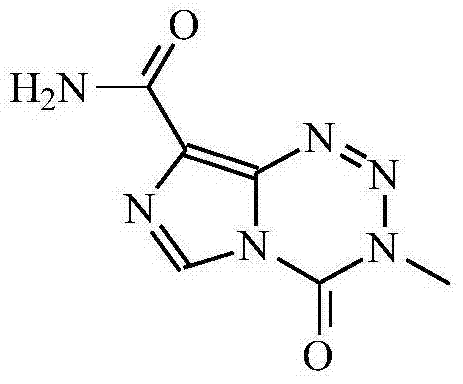
Novel crystal form for temozolomide, method for preparing temozolomide and medicinal composition of temozolomide
A technology of temozolomide crystal and temozolomide, which is applied in the field of medicine, can solve the problems of being unsuitable for industrial production, difficult to meet the quality requirements of injection raw materials, and complicated to operate, and achieve the effects of improving appearance and color, being suitable for long-term storage, and having a simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of Temozolomide M crystal form
[0044] Take 10g temozolomide and place it in the reaction flask, add 50g acetone, 50g acetonitrile and heat up to 50~55℃ under stirring, slowly add 100g purified water dropwise with steady reflux. With the continuous addition of purified water, the solid gradually dissolves. After the addition is complete Slowly cool to 30~40℃ to crystallize for 1 hour, then at 15~25℃ to crystallize for 2 hours, and finally lower the temperature to 5~10℃ to allow the solids to be fully analyzed and crystallized for 5 hours, filtered with suction, and the filter cake is rinsed with water Wash with acetone / water (1:1) mixed solution and drain. The solid was dried under reduced pressure (-0.09MPa) at 45°C to obtain 8.1 g of white solid with a yield of 81%. ; Related substances 0.02%. Tested by powder X-ray diffraction, such as figure 1 . Temozolomide M crystal form.
[0045] HPLC purity detection method: take an appropriate amount of t...
Embodiment 2
[0046] Example 2 Preparation of Temozolomide M crystal form
[0047] Place 10g temozolomide in a reaction flask, add 60g acetone, 60g acetonitrile, stir and heat to reflux, slowly add 40g purified water dropwise with stable reflux. With the continuous addition of purified water, the solid gradually dissolves. After the addition is complete, slowly Cool to 30~40℃ to crystallize for 1 hour, then at 15~25℃ to crystallize for 2 hours, and finally cool to 5~10℃ to allow the solids to be fully analyzed and crystallized for 8 hours, filtered with suction, and the filter cake is rinsed with water and acetone / Water (1:1) mixed solution wash and drain. Phosphorus pentoxide is used as a desiccant for the solid, and it is dried at 45°C under reduced pressure (-0.09MPa) to obtain 8.4g of white solid with a yield of 84% and a purity of 100%. According to powder X-ray diffraction, it is temozolomide M crystal form.
Embodiment 3
[0048] Example 3 Preparation of Temozolomide M crystal form
[0049] Put 5g temozolomide in the reaction flask, add 40g acetone, 20g acetonitrile, stir and heat to reflux, slowly add 30g purified water dropwise with stable reflux, as the purified water is continuously added, the solid gradually dissolves, after the addition, slowly Cool to 30~40℃ and crystallize for 1 hour, then at 15~25℃ for 2 hours, and finally lower the temperature to 5~10℃ to allow the solid to be fully analyzed and crystallized for 10 hours, filtered with suction, and the filter cake is rinsed with water and then washed with acetone / Water (1:1) mixed solution wash and drain. The solid uses phosphorus pentoxide as a desiccant, and is dried under reduced pressure (-0.09MPa) at 45°C to obtain 4.3 g of white solid with a yield of 86% and a purity of 100%. According to powder X-ray diffraction, it is temozolomide M crystal form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com