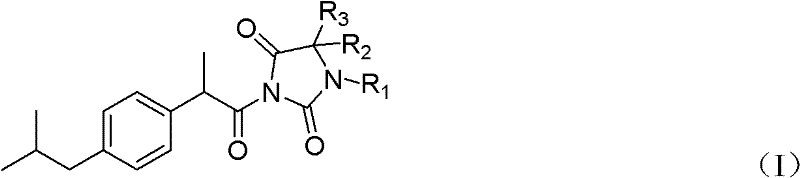
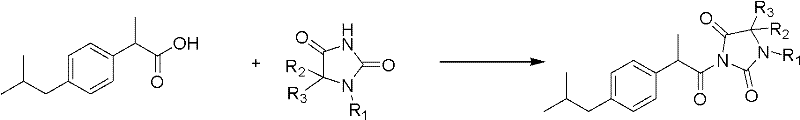
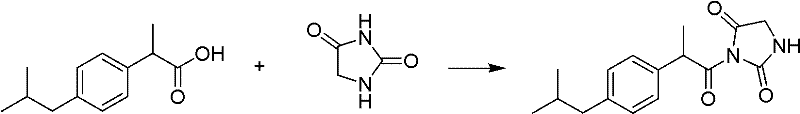
Hydantoin ibuprofen derivative and synthetic method and application thereof
A derivative, propionyl technology, applied in the field of medicine, can solve the problems of ibuprofen ulcer, bleeding, perforation and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of 3-(2-(4-isobutylphenyl) propionyl) imidazolidinyl-2,4-dione (HI-I)
[0027]
[0028] In a 100mL three-necked flask, add 1g of ibuprofen and 5mL of thionyl chloride, heat to reflux for 3h, and evaporate the solution to dryness. Then 0.58 g of hydantoin and 10 mL of pyridine were added to react at room temperature for 12 hours. Evaporation to dryness and column chromatography (methanol:dichloromethane=1:20) gave the product HI-I, 1.12 g of a white solid, with a yield of 80%.
[0029] nuclear magnetic resonance 1 H-NMR (CDCl 3 , 400Hz) δ: 0.87 (d, J=6.64Hz, 6H, 2×CH 3 ), 1.49 (d, J=6.96Hz, 3H, CH 3 ), 1.83(m, 1H, CH), 2.42(d, J=7.16Hz, 2H, CH 2 ), 3.86 (d, J=4.76Hz, 2H, CH 2 ), 4.81(q, J=6.96, 1H, CH), 5.94(s, 1H, NH), 7.07(d, J=8.12Hz, 2H, 2×ArH), 7.15(d, J=8.12Hz, 2H , 2×ArH).
[0030] 13 C-NMR (CDCl 3 , 100Hz) δ: 172.72, 168.52, 154.86, 141.07, 136.43, 129.66, 129.66, 127.61, 127.61, 46.96, 45.71, 44.99, 30.08, 22.35, 22.35, 18.44....
Embodiment 2
[0031] Example 2: Preparation of 1-methyl-3-(2-(4-isobutylphenyl) propionyl) imidazolidinyl-2,4-dione (HI-II)
[0032]
[0033] In a 100mL three-necked flask, add 1g of ibuprofen and 5mL of thionyl chloride, heat to reflux for 3h, and evaporate the solution to dryness. Then 0.67 g of 1-methylhydantoin and 10 mL of pyridine were added, and the mixture was reacted at room temperature for 12 hours. Evaporate to dryness and column chromatography (methanol: methyl chloride = 1:20) to obtain the product HI-II, 1.1 g of a white solid, with a yield of 75%.
[0034] nuclear magnetic resonance 1 H-NMR (CDCl 3 , 400Hz) δ: 0.89 (d, J=6.8Hz, 6H, 2×CH 3 ), 1.49 (d, J=6.8Hz, 3H, CH 3 ), 1.83(m, 1H, CH), 2.42(d, J=7.2Hz, 2H, CH 2 ), 2.93 (s, 3H, CH 3 ), 3.77 (s, 2H, CH 2 ), 4.85 (q, J=6.8Hz, 1H, CH), 7.07 (d, J=8.0Hz, 2H, 2×ArH), 7.16 (d, J=8.0Hz, 2H, 2×ArH).
[0035] 13 C-NMR (CDCl 3 , 100Hz) δ: 172.58, 166.93, 152.86, 140.88, 136.68, 129.57, 129.57, 127.64, 127.64, 50.85, 46.62...
Embodiment 3
[0036] Example 3: Preparation of 1-benzyl-3-(2-(4-isobutylphenyl) propionyl) imidazolidinyl-2,4-dione
[0037]
[0038] In a 100mL three-necked flask, add 1g of ibuprofen and 5mL of thionyl chloride, heat to reflux for 3h, and evaporate the solution to dryness. Then 110 g of 1-benzylhydantoin and 10 mL of pyridine were added, and the mixture was reacted at room temperature for 12 hours. Evaporate to dryness and column chromatography (methanol: dichloromethane = 1:20) to obtain the product HI-II, 1.25 g of oil, yield 68%.
[0039] nuclear magnetic resonance 1 H-NMR (CDCl 3 , 400Hz) δ: 0.89 (d, J=6.56Hz, 6H, 2×CH 3 ), 1.50 (d, J=6.92Hz, 3H, CH 3 ), 1.84(m, 1H, CH), 2.44(d, J=7.16Hz, 2H, CH 2 ), 3.60 (dd, J 1 =17.72Hz,J 2 =25.0Hz, 2H, CH 2 ), 4.46 (dd, J 1 =14.96Hz,J 2 =6.4Hz, 2H, CH 2 ), 4.87 (q, J=6.96Hz, 1H, CH), 7.11 (m, 6H, 6×ArH), 7.32 (m, 3H, 3×ArH).
[0040] 13 C-NMR (CDCl 3 ,100Hz)δ:172.60,166.88,152.84,140.93,136.69,134.42,129.60,129.60,129.02,129.02,12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com