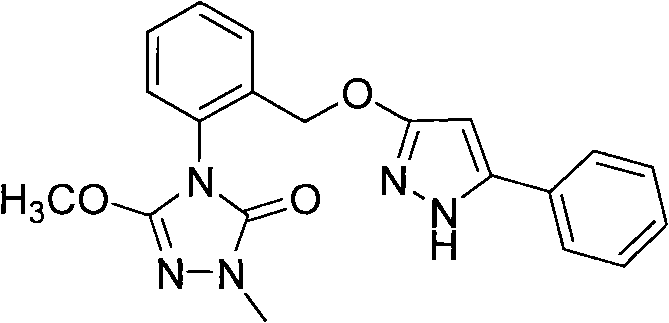
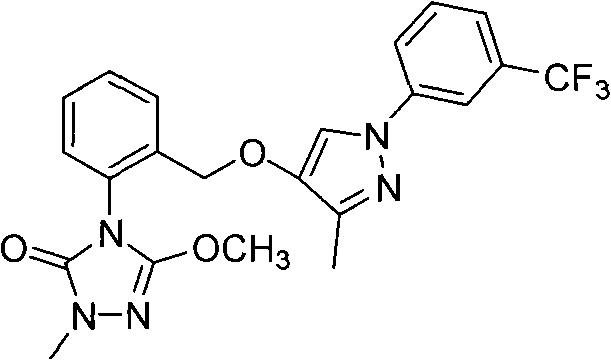
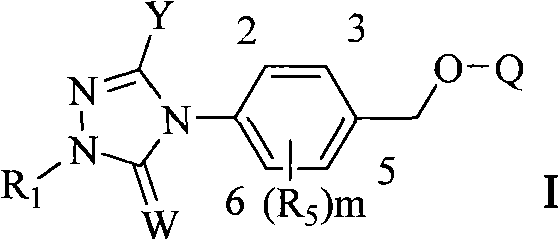
Triazoline ketone ether-substituted compound and application thereof
A technology of triazolone ethers and compounds, which is applied in the field of substituting triazolone ether compounds, and can solve problems such as different structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0133] Example 1: Preparation of Compound 70
[0134]
[0135] 1) Preparation of 4-methylphenylisocyanate
[0136] Weigh 14.85 g (0.05 mol) of triphosgene and put it into a 1000 ml three-neck flask equipped with a mechanical stirrer, a thermometer, and a tail gas absorption device, add 150 ml of 1,2-dichloroethane, and then control the temperature for 0-5 ℃. While stirring at this temperature, 5.35 g (0.05 mol) of p-methylaniline solution (dissolved in 100 ml of 1,2-dichloroethane) was slowly added dropwise. A large amount of insoluble matter appeared during the dropwise addition. After dropping, the temperature of the reaction solution was raised to reflux for reaction. After the reaction solution was clarified, the reaction was completed, and the reaction solution was precipitated to obtain 6.52 grams of a red oily substance.
[0137] 2) Preparation of intermediate 2,2-dimethyl-N-(4-methylphenyl)hydrazide
[0138] Dissolve 10 g of 4-methylphenylisocyanate in 75 ml of ...
example 2
[0146] Example 2: Preparation of Compound 500
[0147]
[0148] 1) Preparation of intermediate 2,4-dihydro-5-methoxy-2-methyl-4-(4-methylphenyl)-3H-1,2,4-triazol-3-one
[0149] Dissolve 8.25 g of 5-chloro-2,4-dihydro-2-methyl-4-(4-methylphenyl)-3H-1,2,4-triazol-3-one in 80 mL of methanol 14.0 ml of 30% (mass fraction) sodium methoxide / methanol solution was added, and the reaction was refluxed for 3 hours. After the completion of the reaction as monitored by TLC, the reaction solution was poured into 100 milliliters of saturated brine, extracted with ethyl acetate, the extract was dried with anhydrous magnesium sulfate and precipitated under reduced pressure, column chromatography (the volume ratio of ethyl acetate and sherwood oil was 1: 5) Purified to obtain 5.2 g of white solid.
[0150] 2) Preparation of Intermediate II-2
[0151] Dissolve 6.7 g of 2,4-dihydro-5-methoxy-2-methyl-4-(4-methylphenyl)-3H-1,2,4-triazol-3-one in 100 ml Add 6.53 g of NBS and 0.05 g of azobi...
Embodiment 3
[0162] Example 3: 30% Compound 84 wettable powder
[0163] Compound 84 30%
[0164] Sodium Lauryl Sulfate 2%
[0165] Sodium lignosulfonate 3%
[0166] Naphthalenesulfonic acid formaldehyde condensate 5%
[0167] Light Calcium Carbonate Top up to 100%
[0168] Compound 84 and other components were fully mixed, and pulverized by a superfine pulverizer to obtain a 30% wettable powder product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com