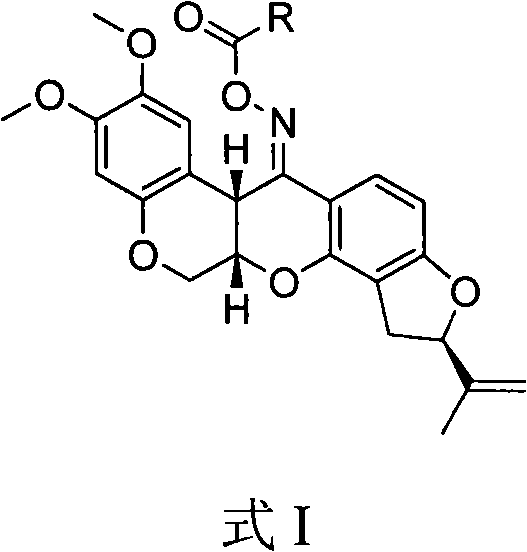
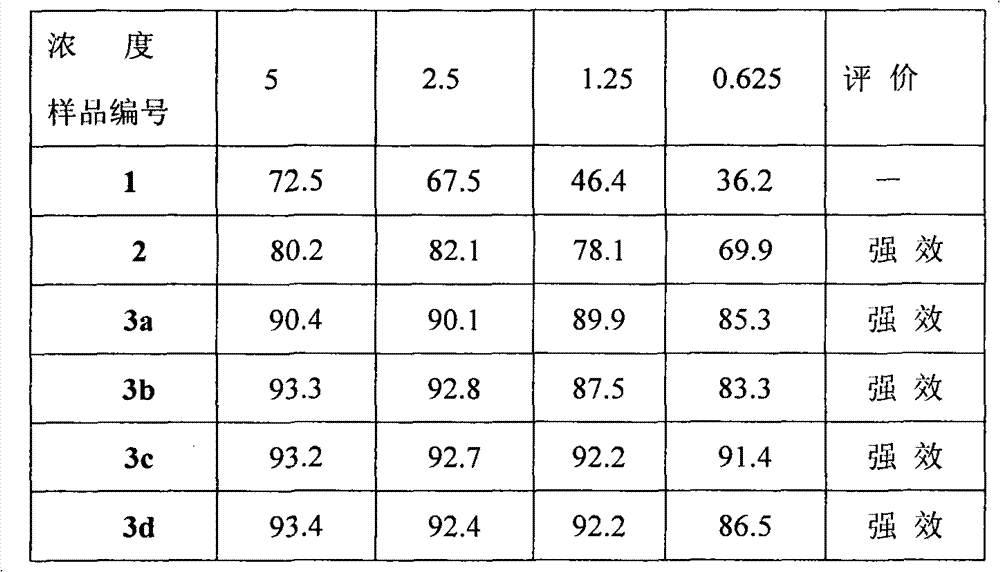
Spin-labeling rotenone oxime ester, its preparation method and application
A technology of rotenone oxime and spin labeling, which is applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve problems such as unsatisfactory market development needs, limited resources, failure of chemotherapy, etc., and achieve excellent development and application prospects, and synthesis process Simple, high product purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
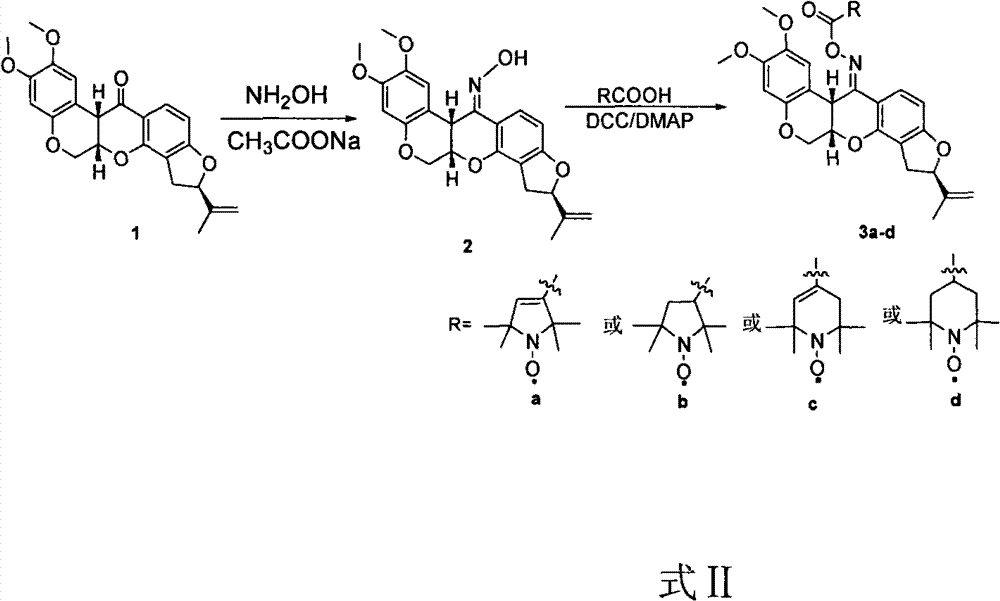
[0019] Synthesis of 12-N-[2′, 2′, 5′, 5′-tetramethyl-pyrroline nitroxide radical-3′-acyloxy]-rotenone oxime ester (compound 3a)
[0020] Synthesis of raw material rotenone oxime (2): 2mmol rotenone, 2.5mmol hydroxylamine and 2.5mmol sodium acetate were mixed, then 200mL ethanol was added, and refluxed for 12 hours. Crystallization appeared, filtered, washed with water, and dried to obtain the crude product. For the method used, refer to the method reported in the literature Yang Shiping, et al. Crystal Structure Communications, 2003, C59, 392-393.
[0021] Dissolve 1 mmol of rotenone oxime in 30 mL of dichloromethane, add 1.5 mmol of 3-carboxylic acid-2,2,5,5-tetramethylpyrroline nitroxide radical, and add 0.5 mmol of N, N -lutidine, and 1.5 mmol of dicyclohexylcarbodiimide was added, stirred at room temperature under argon protection for 5 hours, filtered to remove the white precipitate, and the solvent was removed under reduced pressure, the crude product was purified by co...
Embodiment 2
[0024] Synthesis of 12-N-[2′,2′,5′,5′-tetramethyl-tetrahydropyrroline nitroxide radical-3′-acyloxy]-rotenone oxime ester (compound 3b)
[0025] The experimental procedure is the same as in Example 1, only 3-carboxylic acid-2,2,5,5-tetrahydropyrroline nitroxide radical is used to replace 3-carboxylic acid-2,2,5,5-tetramethyl ylpyrroline nitroxide free radical. The detection data of the product obtained from the reaction are as follows: Yield: 82%; m.p.116-117°C; IR (KBr) cm -1 : 3436, 2973, 1763, 1621, 1513, 1458, 1355, 1217, 1080; ESR: An=14.76G, g 0 =2.0058; HRMS m / z calcd for C 32h 37 N 2 o 8 : 578.2623[M+H] + , found 578.2636[M+H] + .
Embodiment 3
[0027] Synthesis of 12-N-[2′, 2′, 6′, 6′-tetramethyl-tetrahydropyridine nitroxide radical-4′-acyloxy]-rotenone oxime ester (compound 3c)
[0028] The experimental procedure is the same as in Example 1, only 4-carboxylic acid-2,2,6,6-tetrahydropyridine nitroxide radical is used to replace 3-carboxylic acid-2,2,5,5-tetramethyl ylpyrroline nitroxide free radical. The detection data of the product obtained from the reaction are as follows:: Yield: 68%; m.p.124-126°C; IR (KBr) cm -1 : 3449, 2974, 1741, 1622, 1513, 1458, 1354, 1218, 1042; ESR: An = 15.28G, g 0 =2.0064; HRMS m / z calcd for C 33 h 37 N 2 o 8 : 590.2623[M+H] + , found 590.2638[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com