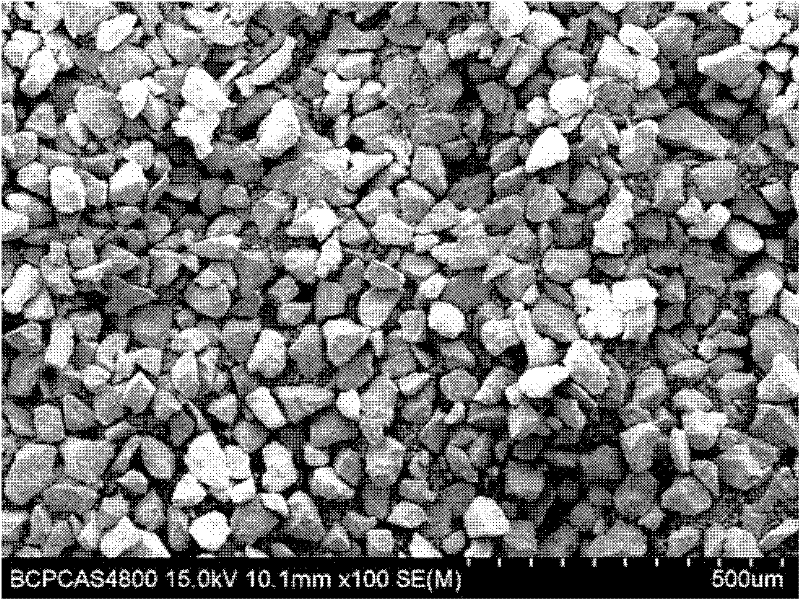Rapidly synthesized aluminate long-afterglow luminescent material and preparation method thereof
A technology of long afterglow luminescence and long afterglow materials, which is applied in the direction of luminescent materials, chemical instruments and methods, etc. It can solve the problems of long heating time, energy waste, and coarse product particles, and achieve the improvement of crystal phase purity, increase luminous intensity, and precipitation fine and uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
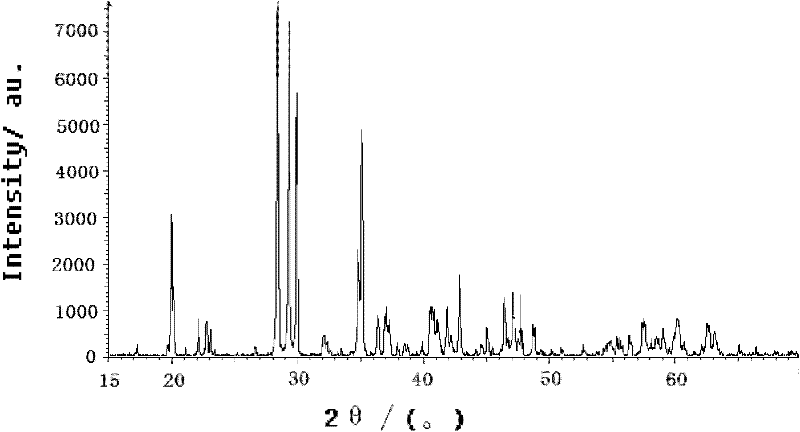
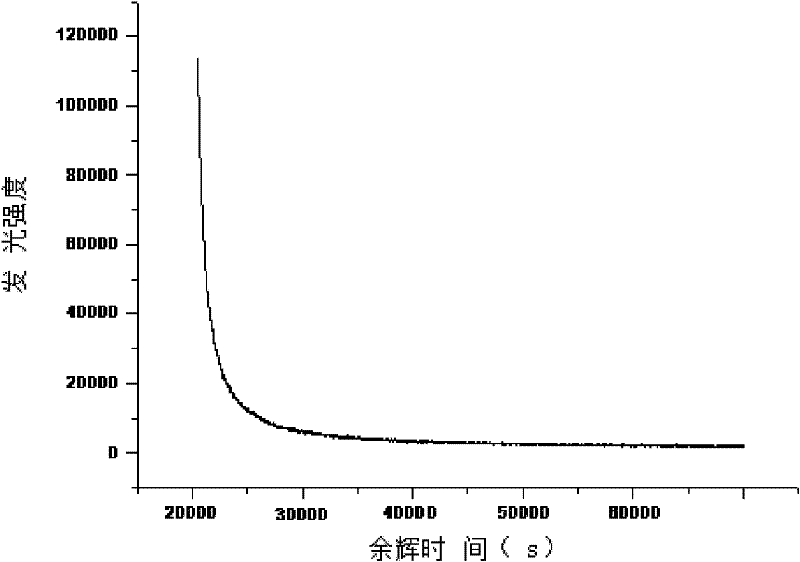
[0017] According to the general formula Sr (1-x-y) Al 2 o 4 :Eu x , Dy y , where x=0.001, y=0.01, first weigh ①Sr(NO 3 ) 2 20.845g, ②Al(NO 3 ) 3 9H 2 O 75.022g, ③Eu(NO 3 ) 3 ·6H 2 O 0.139g, ④Dy(NO 3 ) 3 ·6H 2 O 0.273g, respectively dissolved with deionized water, wherein the solid-to-liquid ratio is 1:1~2; after mixing the solutions, add to 800ml (NH 2 ) 2 CO solution, the (NH 2 ) 2 The concentration of the CO solution is 0.8mol / L, the molar ratio of the added amount of the urea solution to the soluble material is 8:1, then heated to 96°C and stirred to form a precipitate, and the precipitate is separated from the liquid; Washed twice with deionized water, then dried the washed product by microwave drying, and dried at 600W for 30min to obtain composite precursor fractions with fine and uniform particle size. Then at 90% N 2 ~10%H 2 Under a mixed atmosphere, sinter in a microwave sintering furnace at 3000W for 15min to obtain Sr 0.989 Eu 0.001 Dy 0.01 Al...
Embodiment 2
[0019] According to the general formula Sr (1-x-y) Al 2 o 4 :Eu x , Dy y , where x=0.015, y=0.1, first weigh ①Sr(NO 3 ) 2 216.697g, ②Al(NO 3 ) 3 9H 2 O 75.022g, ③Eu(NO 3 ) 3 ·6H 2 O 0.07g, ④Dy(NO 3 ) 3 ·6H 2 O0.137g, dissolve respectively with deionized water; Wherein the solid-liquid ratio is 1: 1~2; After mixing each solution again, add to 600ml (NH 2 ) 2 CO solution, the (NH 2 ) 2 The concentration of the CO solution is 0.4mol / L, the molar ratio of the amount of the urea solution added to the soluble material is 5:1, then heated to 96°C and stirred to form a precipitate, and the precipitate is separated from the liquid; then the precipitate is used Washed twice with deionized water, then dried the washed product by microwave drying, and dried at 600W for 30min to obtain composite precursor fractions with fine and uniform particle size. Then at 90% N 2 ~10%H 2 Under a mixed atmosphere, sinter in a microwave sintering furnace at 2500W for 20min to obtain S...
Embodiment 3
[0021] According to the general formula Sr (1-x-y) Al 2 o 4 :Eu x , Dy y , where x=0.03, y=0.2, first weigh ①Sr(NO 3 ) 2 18.411g, ②Al(NO 3 ) 3 9H 2 O 75.022g, ③Eu(NO 3 ) 3 ·6H 2 O 0.105g ④Dy(NO 3 ) 3 ·6H 2 O0.206g, dissolve respectively with deionized water; Wherein the solid-liquid ratio is 1: 1~2; After mixing each solution again, add to 600ml (NH 2 ) 2 CO solution, the (NH 2 ) 2 The concentration of the CO solution is 0.6mol / L, the molar ratio of the added amount of the urea solution to the soluble material is 6:1, then heated to 96°C and stirred to form a precipitate, and the precipitate is separated from the liquid; Washed twice with deionized water, then dried the washed product by microwave drying, and dried at 600W for 30min to obtain composite precursor fractions with fine and uniform particle size. Then at 90% N 2 ~10%H 2 Under a mixed atmosphere, sinter in a microwave sintering furnace at 2000W for 25min to obtain Sr 0.77 Eu 0.03 Dy 0.2 Al 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com