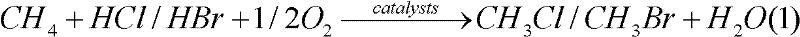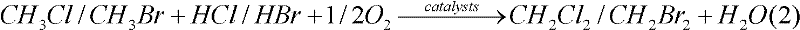Application of cerium-based catalyst in methane oxyhalogenation methods used for preparing halogenated methane
A cerium-based catalyst, methane halogen oxidation technology, applied in physical/chemical process catalysts, carbon monoxide, halogenated hydrocarbon preparation and other directions, can solve the problems of easy deliquescence and complex preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Catalyst preparation:
[0033] 3.47g Ce(NO 3 ) 2 *6H 2 Dissolve O in 20 mL of deionized water, add 140 ml of 9M NaOH solution under stirring, then transfer to an autoclave for 24 h at 100 ° C, cool, filter with suction, dry at 100 ° C, and roast at 550 ° C for 6 h to obtain CeO 2 .
[0034] Catalyst Test:
[0035] The reaction was carried out in a fixed-bed flow reactor at normal pressure, and the reactor was a quartz glass tube with an inner diameter of 10 mm. The reaction conditions are: catalyst dosage is 0.500g, reaction temperature is 480°C, CH 4 Flow 16.0mL / min, HCl flow 8.0mL / min, O 2 The flow rate is 8.0mL / min. The tail gas was analyzed by gas chromatography, with N 2 Do internal standard.
[0036] Experiments show that the conversion rate of methane is 27%, the selectivity of monochloromethane is 55%, the selectivity of dichloromethane is 14%, the selectivity of chloroform is 0.3%, the selectivity of carbon monoxide is 15%, and the selectivity of carbo...
Embodiment 2
[0038] Catalyst preparation:
[0039] 3.47g Ce(NO 3 ) 2 *6H 2 Dissolve O in 20 mL of deionized water, add 140 ml of 6M NaOH solution under stirring, then transfer to an autoclave for 24 h at 180 ° C, cool, filter with suction, dry at 100 ° C, and roast at 600 ° C for 6 h to obtain CeO 2 .
[0040] Catalyst Test:
[0041] The reaction was carried out in a fixed-bed flow reactor at normal pressure, and the reactor was a quartz glass tube with an inner diameter of 20 mm. The reaction conditions are: catalyst dosage is 2.000g, reaction temperature is 600°C, CH 4 Flow 10.0mL / min, O 2 Flow rate 5.0mL / min, 40wt% HBr / H 2 The O flow rate is 4.0 mL / min. Tail gas and liquid products were analyzed on a gas chromatograph.
[0042] Experiments show that the conversion rate of methane is 18%, the selectivity of methyl bromide is 75%, the selectivity of dibromomethane is 3.2%, the selectivity of carbon monoxide is 20%, and the selectivity of carbon dioxide is 1.4%.
Embodiment 3~8
[0044] Catalyst preparation:
[0045] 3.47g Ce(NO 3 ) 2 *6H 2 O and the corresponding metal nitrate solution were dissolved in 20mL of deionized water according to the mol composition of the catalyst in Table 1. After mixing evenly, 140ml of 9M NaOH solution was added under stirring, and then transferred to an autoclave for 24h at 100°C, cooled, and pumped. Filter, dry at 100°C, and calcined at 550°C for 6h.
[0046] Catalyst Test:
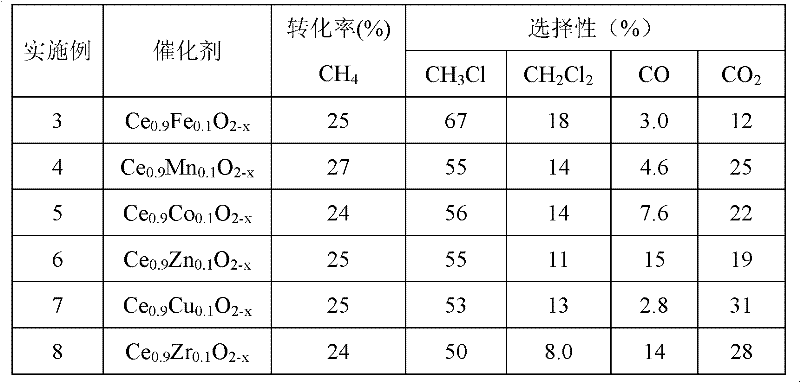
[0047] According to the test conditions in Example 1, see Table 1 for the experimental results of Examples 3-8.
[0048] Table 1
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com