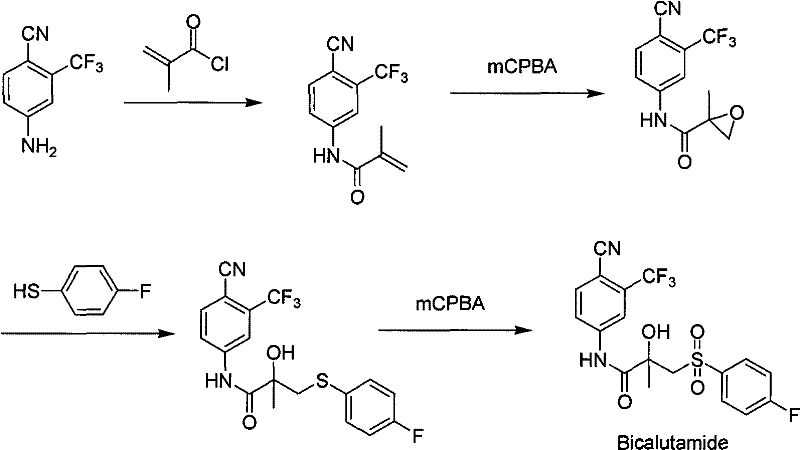
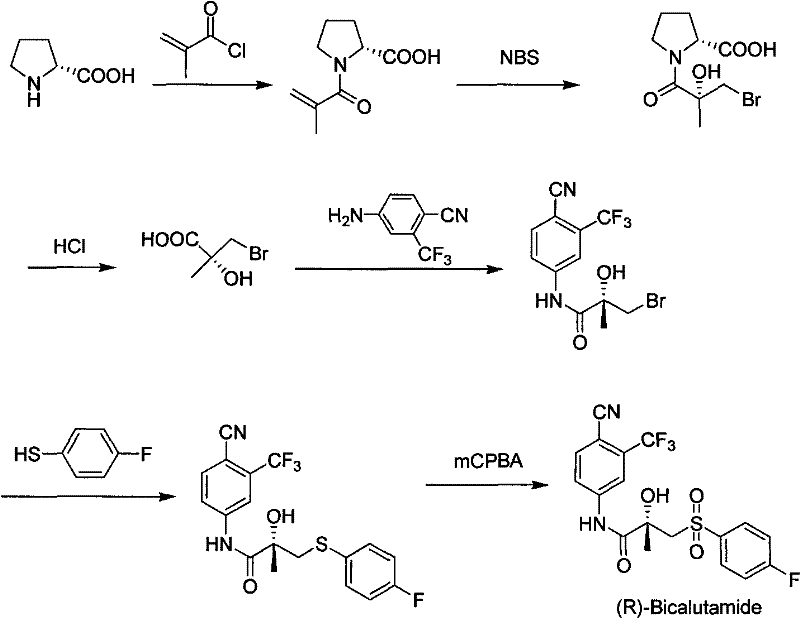
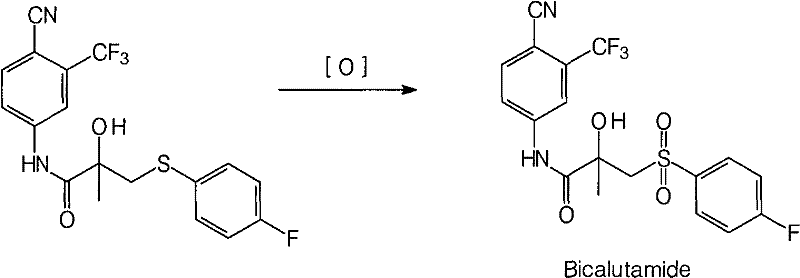
Method for preparing bicalutamide from N-(4-cyano-3-trifluoromethylphenyl)-3-(4-fluorophenylsulfenyl)-2-hydroxy-2-methyl-propanamide
A technology of trifluoromethylphenyl and hydroxypropionamide, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as high production cost, environmental pollution, and complicated treatment, and achieve low cost , Reduce environmental pollution, easy to react
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Compound 1 (10.0g, 0.025mol) was dissolved in acetone (30ml), sodium bicarbonate (0.1g, 0.00125mol) was added, and the reaction temperature was slowly added dropwise at 0°C with a concentration of 30% aqueous hydrogen peroxide ( 2.8g, 0.025mol), keep the temperature for 24h. The reaction solution was poured into water (100ml) and stirred for 30min. After filtration, the filter cake was washed with water, and air-dried at 50°C to obtain 4.1 g of white bicalutamide solid, with a yield of 38.2%, a melting point of 190-193°C, and an HPLC content of 97.8%.
Embodiment 2
[0033] Compound 1 (10.0g, 0.025mol) was dissolved in acetone (50ml), sodium bicarbonate (0.1g, 0.00125mol) was added, and the reaction temperature was slowly added dropwise at 0°C with a concentration of 30% aqueous hydrogen peroxide ( 2.8g, 0.025mol), keep the temperature for 24h. The reaction solution was poured into water (100ml) and stirred for 30min. After filtration, the filter cake was washed with water, and air-dried at 50°C to obtain 4.2 g of white bicalutamide solid, with a yield of 39.3%, a melting point of 190-193°C, and an HPLC content of 98.0%.
Embodiment 3
[0035] Compound 1 (10.0g, 0.025mol) was dissolved in acetone (80ml), sodium bicarbonate (0.1g, 0.00125mol) was added, and the reaction temperature was slowly added dropwise at 0°C with a concentration of 30% aqueous hydrogen peroxide ( 2.8g, 0.025mol), keep the temperature for 24h. The reaction solution was poured into water (100ml) and stirred for 30min. After filtration, the filter cake was washed with water, and air-dried at 50°C to obtain 3.8 g of white bicalutamide solid, with a yield of 35.3%, a melting point of 190-193°C, and an HPLC content of 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com