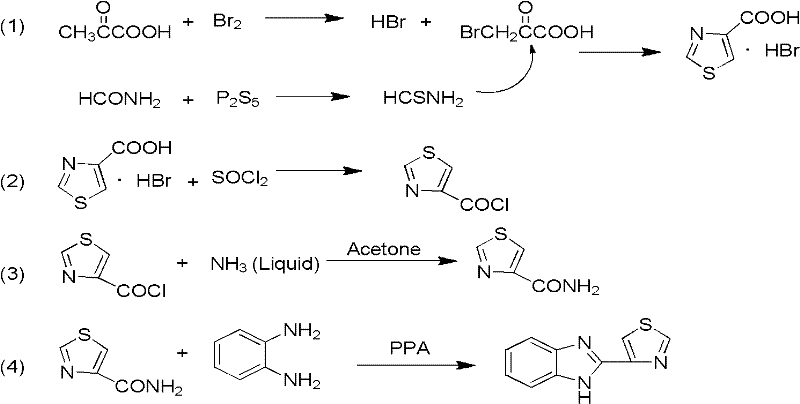
Probenazole low-toxicity bactericide and preparation method thereof
A technology of thiabendazole and bactericide, which is applied in the field of low toxicity bactericide thiabendazole and its preparation, can solve the problems of potential safety hazards, increased cost and risk, and expensive raw materials of pyruvic acid, etc., so as to improve the conversion rate and yield , Simplify the production process and reduce the effect of unsafe factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
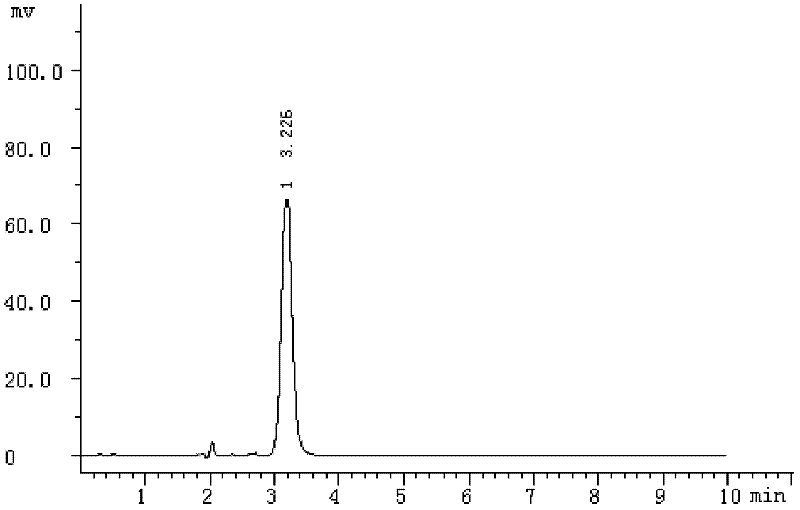
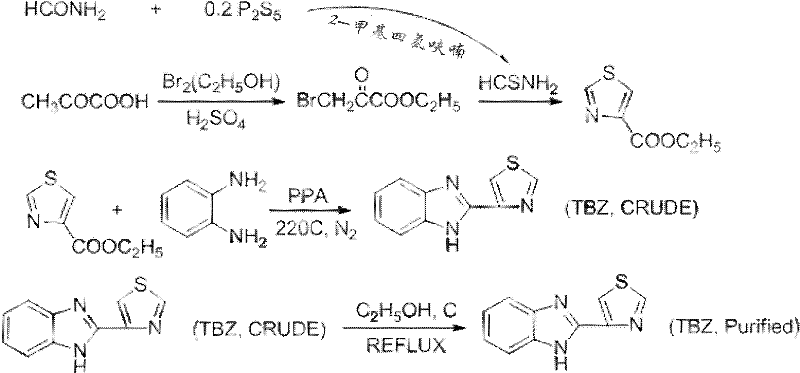
[0042] A preparation method of low-toxicity bactericide thiabendazole, the steps are as follows:
[0043] (1) 27.2g of bromine was dissolved in 40ml of ethanol while cooling in a water bath to obtain solution A;
[0044] (2) Add 8.8 g of pyruvic acid into 1 ml of concentrated sulfuric acid, heat to 50° C., then add the solution A prepared in step (1), and react for 2.0 h to obtain mixture I;
[0045] (3) Add 5g P 2 S 5 Add 5g of formamide into 213.5g of 2-methyltetrahydrofuran, and react at 50°C for 1.5h to obtain solution II;
[0046] (4) Add the mixture I prepared in step (2) to solution II, raise the temperature to 70°C, react for 4h, cool to room temperature, let stand for 10h, filter to collect the precipitate, and dry at 60°C to obtain thiazole-4- ethyl carboxylate;
[0047] (5) Add 2.1 g of o-phenylenediamine and 5 g of ethyl thiazole-4-carboxylate prepared in step (4) to 50 g of polyphosphoric acid, mix well and heat to 150° C. under the protection of nitrogen, and...
Embodiment 2
[0070] A preparation method of low-toxicity bactericide thiabendazole, the steps are as follows:
[0071] (1) 22g of bromine was dissolved in 40ml of ethanol while cooling in a water bath to obtain solution A;
[0072] (2) Add 8.8 g of pyruvic acid into 1 ml of concentrated sulfuric acid, heat to 50° C., then add the solution A prepared in step (1), and react for 2.0 h to obtain mixture I;
[0073] (3) Add 4g P 2 S 5 Add 5g of formamide into 213.5g of 2-methyltetrahydrofuran, and react at 50°C for 1.5h to obtain solution II;
[0074] (4) Add the mixture I prepared in step (2) to solution II, raise the temperature to 70°C, react for 4h, cool to room temperature, let stand for 10h, filter to collect the precipitate, and dry at 60°C to obtain thiazole-4- ethyl carboxylate;
[0075] (5) Add 1.5 g of o-phenylenediamine and 5 g of ethyl thiazole-4-carboxylate prepared in step (4) to 45 g of polyphosphoric acid, mix well and heat to 150° C. under the protection of nitrogen, and r...
Embodiment 3
[0079] A preparation method of low-toxicity bactericide thiabendazole, the steps are as follows:
[0080] (1) Dissolve 30.8g of bromine in 40ml of ethanol while cooling in a water bath to obtain solution A;
[0081] (2) Add 8.8 g of pyruvic acid into 1 ml of concentrated sulfuric acid, heat to 50° C., then add the solution A prepared in step (1), and react for 2.0 h to obtain mixture I;
[0082] (3) Add 6g P 2 S 5 Add 5ml of formamide into 213.5g of 2-methyltetrahydrofuran and react at 50°C for 1.5h to obtain solution II;
[0083] (4) Add the mixture I prepared in step (2) to solution II, raise the temperature to 70°C, react for 4h, cool to room temperature, let stand for 10h, filter to collect the precipitate, and dry at 60°C to obtain thiazole-4- ethyl carboxylate;
[0084] (5) Add 3.0 g of o-phenylenediamine and 5 g of ethyl thiazole-4-carboxylate prepared in step (4) to 55 g of polyphosphoric acid, mix well and heat to 150° C. under the protection of nitrogen, and reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com