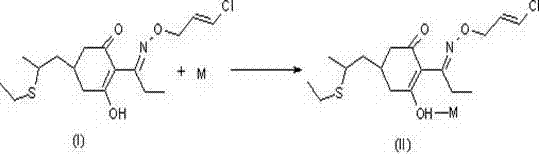
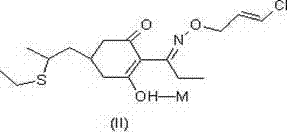
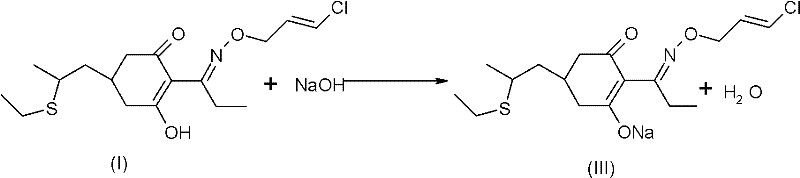
Method for improving stability of clethodim
A technology for clethodim and stability, applied in the field of improving the stability of clethodim, can solve problems such as reducing the reaction yield and increasing the preparation cost, and achieves the effects of high reaction yield, suitability for industrial production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 clethodim methylamine salt
[0021] Add 36g of clethodim technical substance (86%, 0.086mol) and 90g of n-hexane into the reaction flask, and stir evenly at room temperature. Under cooling conditions, slowly introduce methylamine gas. Control the temperature at 0-5°C, and stir at 20°C for 1.5 hours after the addition. The reaction was filtered to give an off-white solid. After drying under reduced pressure, 34 g of the product was obtained with a melting point of 131°C. After detection and analysis by high-pressure liquid chromatography (HPLC), the converted clethodim content is 95.1%, and the reaction yield is 96.2%.
Embodiment 2
[0022] The preparation of embodiment 2 clethodim ethylamine salt
[0023] Add 36g of clethodim technical substance (86%, 0.086mol) and 80g of petroleum ether into the reaction flask, and stir evenly at room temperature. Under cooling conditions, slowly introduce ethylamine gas. Control the temperature at 5-10°C, and stir at 20°C for 1.5 hours after the addition. The reaction was filtered to give an off-white solid. After drying under reduced pressure, 35 g of the product was obtained, with a melting point of 137° C., detected and analyzed by high-pressure liquid chromatography (HPLC), and the converted clethodim content was 95.1%, and the reaction yield was 95.9%.
Embodiment 3
[0024] The preparation of embodiment 3 clethodim propylamine salt
[0025] Add 36g of clethodim technical substance (86%, 0.086mol) and 70g of cyclohexane into the reaction flask, and stir evenly at room temperature. Under cooling conditions, slowly add propylamine liquid dropwise. Control the temperature at 10-20°C, and stir at 20°C for 1.5 hours after the addition. The reaction was filtered to give an off-white solid. After drying under reduced pressure, 36 g of the product was obtained, with a melting point of 142°C. After detection and analysis by high-pressure liquid chromatography (HPLC), the converted clethodim content is 95.1%, and the reaction yield is 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com