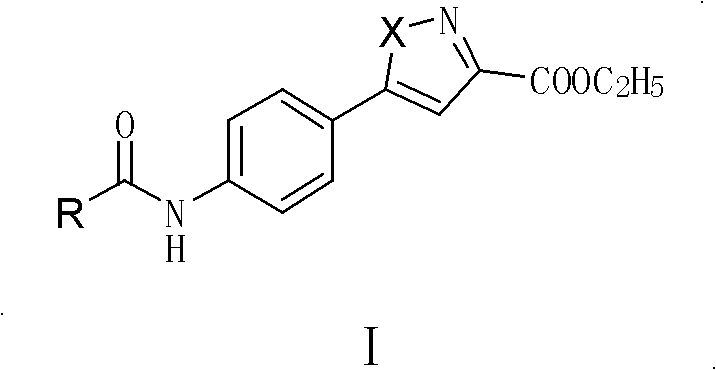
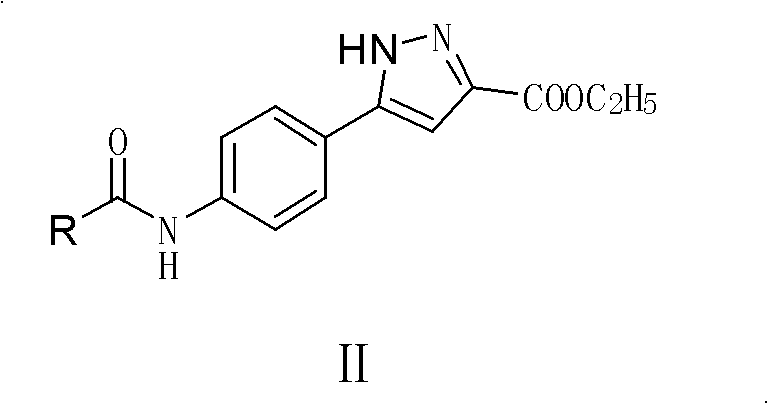
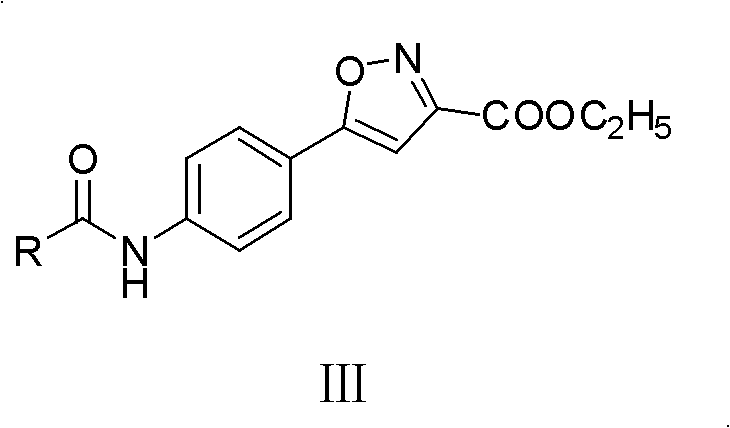
Parazole and isoxazole derivative, preparation method for same and application thereof
A technology of isoxazole and derivatives, applied in the field of pharmaceutical compounds, can solve the problems of poor specificity and low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0125] Synthesis of 5-(4-substituted arylamide)-1H-pyrazole-3-carboxylic acid ethyl ester II compound (compound II-1);
[0126] (1) Synthesis of 4-(4-nitro)phenyl-2,4-dioxo-butyric acid ethyl ester (intermediate 2)
[0127] In a dry reaction flask, add 400 mL of absolute ethanol and 2.3 g of sodium metal to prepare a sodium ethoxide solution. Add 8.3g of p-nitroacetophenone to the sodium ethoxide solution, stir at room temperature until the solid is completely dissolved, then add 15.74g of diethyl oxalate, react for 6 hours, add acetic acid to adjust the pH to 6.5, and filter with suction to obtain a yellow solid, which is Intermediate 2, dried to obtain 11.7g;
[0128] (2) Synthesis of ethyl 5-(4-nitrophenyl)-1H-pyrazole-3-carboxylate (intermediate 3)
[0129] Intermediate 2 (11.7g, 0.044mol) and hydrazine hydrate (2.6mL, 0.044mol) with a weight concentration of 85% were added to 400% ethanol solution, refluxed for 6 hours, allowed to stand, and filtered to obtain Intermedi...
Embodiment 6~16
[0136] Preparation of Compound II-8:
[0137] Intermediates 2-4 were prepared according to steps (1)-(3) in Example 1, except for step (4);
[0138] (4) Synthesis of target compound 5-(4-benzamidophenyl)-1H-pyrazole-3-carboxylic acid ethyl ester (II-8)
[0139] Compound 4 (0.58g, 2.5mol) was added to 50mL of anhydrous ethyl acetate solution, stirred at room temperature until the solid was completely dissolved, then 1.5g of triethylamine was added, and benzoyl chloride (2.5 mol) was reacted for 6 hours, washed with water, and recrystallized from methanol to obtain 0.25 g of a white solid.
[0140] In the 5-(4-substituted arylamide)-1H-pyrazole-3-carboxylic acid ethyl ester compounds of the present invention, II-3, II-4, II-8, II-9 to II-21 are all according to the above prepared in the same way, only using different acid chlorides in step (4), the acid chlorides are respectively n-butyryl chloride, n-pentanoyl chloride, benzoyl chloride, p-fluorobenzoyl chloride, 2,6-difluoro...
Embodiment 17~33
[0142] Synthesis of 5-(4-substituted arylamide)-isoxazole-3-carboxylic acid ethyl ester III compound (III-1):
[0143] (1) Synthesis of 4-(4-nitro)phenyl-2,4-dioxo-butyric acid ethyl ester (intermediate 2)
[0144] In a dry reaction flask, add 400 mL of absolute ethanol and 2.3 g of sodium metal to prepare a sodium ethoxide solution. Add 8.3g of p-nitroacetophenone to the sodium ethoxide solution, stir at room temperature until the solid is completely dissolved, then add 15.74g of diethyl oxalate, react for 6 hours, add acetic acid to adjust the pH to 6, and filter with suction to obtain a yellow solid, which is the intermediate Body 2, dried to obtain 11.7g;
[0145] (2) Synthesis of ethyl 5-(4-nitrophenyl)-1H-pyrazole-3-carboxylate (intermediate 31)
[0146] Intermediate 2 (11.7g, 0.044mol) and hydroxylamine hydrochloride (3.1g, 0.044mol) were added to 400mL of absolute ethanol, refluxed for 6 hours, and filtered to obtain intermediate 3' (7.2g);
[0147] (3) Synthesis of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com