Preparation method of docetaxel
A technology of docetaxel and compounds, which is applied in the field of preparation of anti-cancer raw material drug docetaxel, can solve the problems of high cost, difficult reduction of product price, and low yield of docetaxel technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
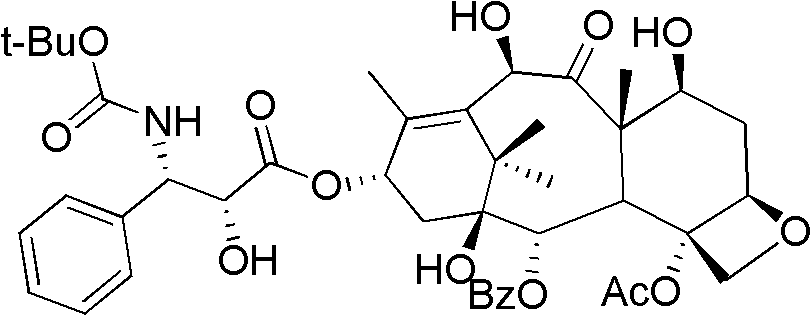
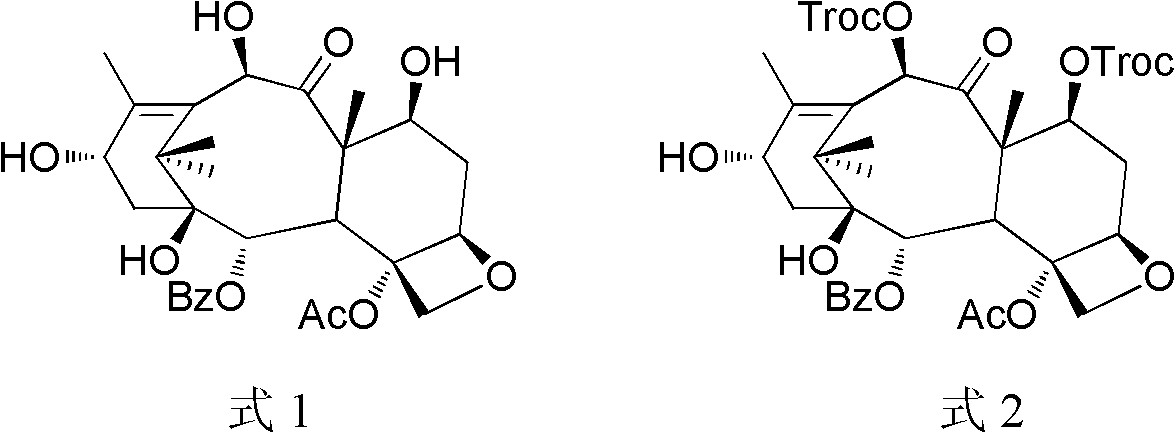
[0036] Dissolve 2.0 g of 10-DABIII in 20 mL of pyridine, add 1.56 mL of trichloroethyl chloroformate, react at zero temperature for 1 hour after the addition, and the reaction is complete. The reaction was quenched with ice-water mixture and extracted with dichloromethane. Dry over anhydrous sodium sulfate and concentrate. Compound 1 was obtained.
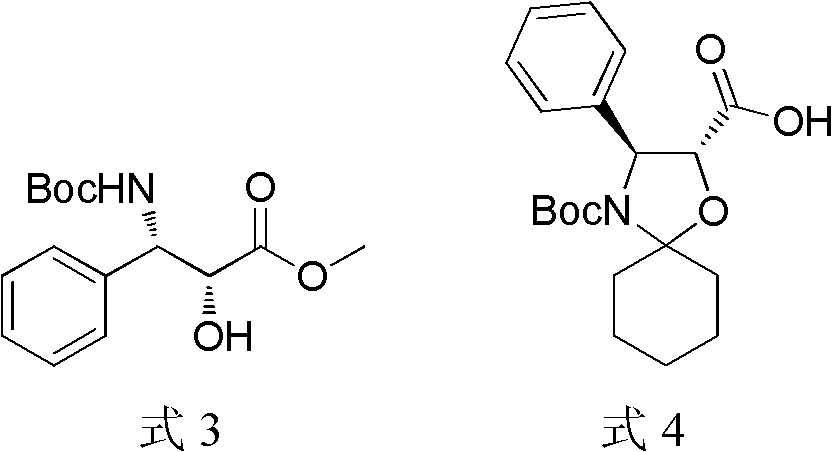
[0037] Dissolve 3 grams of side chains in 15 mL of dichloromethane, add dropwise 2.4 mL of 1,1-dimethoxycyclohexane, add 0.3 mL of boron trifluoride ether, and react at room temperature. After the reaction was complete, the solvent was drained, added with saturated sodium bicarbonate solution, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and then column chromatographed. 1.0 g of the obtained compound was dissolved in 10 mL of tetrahydrofuran, and 5.3 mL of 1.0 N lithium hydroxide solution was slowly added dropwise. After the addition was complete, the reaction was co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com