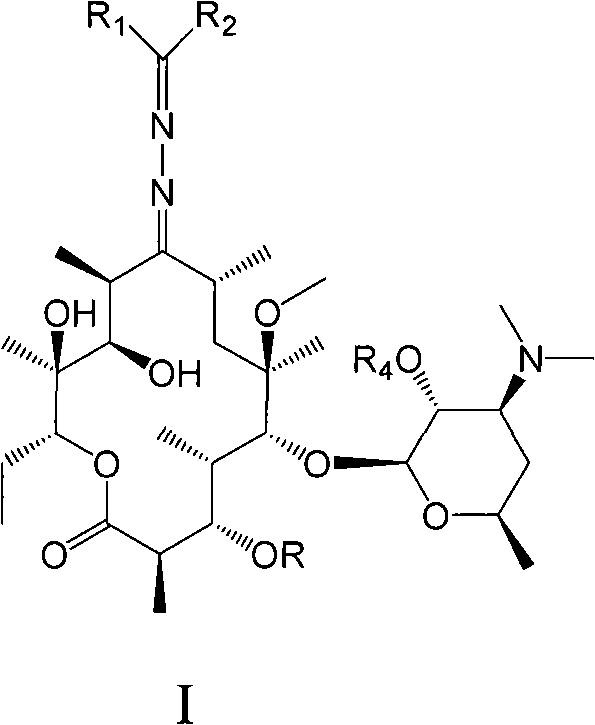
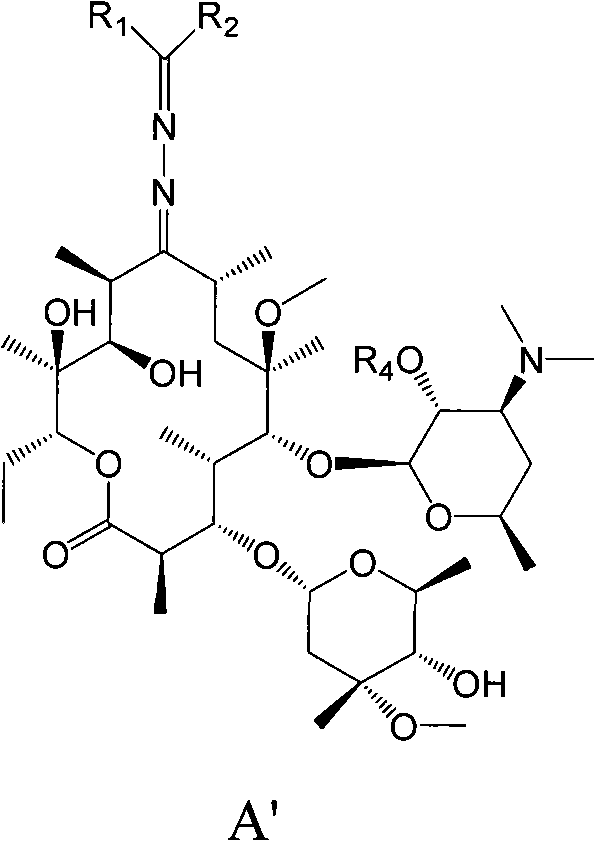
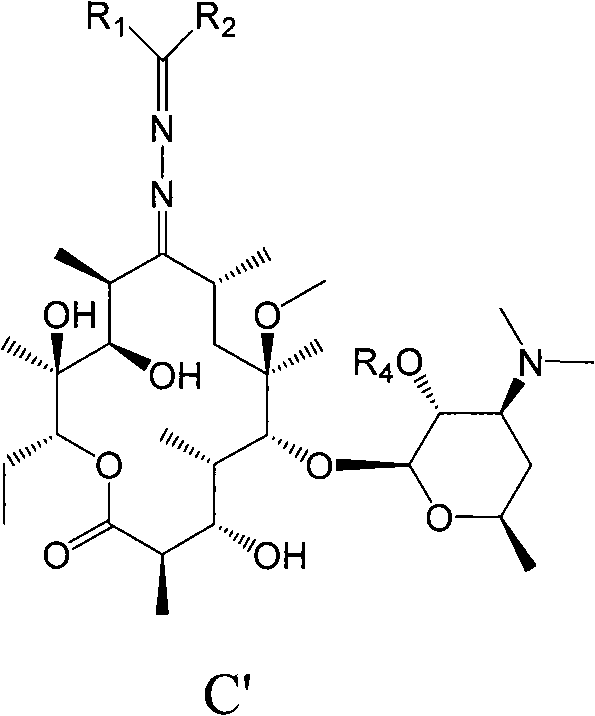
Erythromycin A derivative and preparation method thereof
A technology of erythromycin and its derivatives, which is applied in the field of erythromycin A derivatives and its preparation, and can solve the problems of poor activity of drug-resistant bacteria and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 9-hydrazone erythromycin thiocyanate A (intermediate 12)
[0111] Erythromycin thiocyanate A (40g, 0.0504mol) was dissolved in methanol (40ml), and 85% hydrazine hydrate (4.3ml, 0.0757mol) was added, heated to reflux for 18h and then cooled to precipitate a solid, filtered, and the filter cake was washed with water to obtain a white solid Intermediate 12 (27.1 g, 66.6%).
Embodiment 2
[0112] Example 2 9-hydrazone erythromycin A (intermediate 13)
[0113] 9-hydrazone erythromycin thiocyanate A (20g, 0.0248mol) was dissolved in dichloromethane (400ml)-water (400ml), adjusted to pH 9-10 with 3mol / L sodium hydroxide solution, separated the organic phase, After washing with water, drying with anhydrous sodium sulfate, and filtering, the filtrate was evaporated to dryness under reduced pressure to obtain intermediate 13 (14.0 g, 75.7%) as a white foam.
[0114] MS (ESI + , m / e): 748 [M+H] +
Embodiment 3
[0115] Example 3 9-(propane-2-ylidene hydrazono)erythromycin A (intermediate 15)
[0116] 9-Hydrazone erythromycin A (5.00g, 6.69mmol) was dissolved in acetone (15ml), heated to reflux for 2h and then cooled. A solid precipitated and was filtered to give white solid intermediate 15 (4.70g, 89.2%).
[0117] MS (ESI + , m / e): 788 [M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com