Method for separating rare earth ions by extraction of liquid-liquid-liquid three-phase system
A rare earth ion and three-phase system technology, applied in the direction of improving process efficiency, etc., to achieve the effects of easy artificial control, easy hydrophilicity and hydrophobicity, and difficult emulsification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
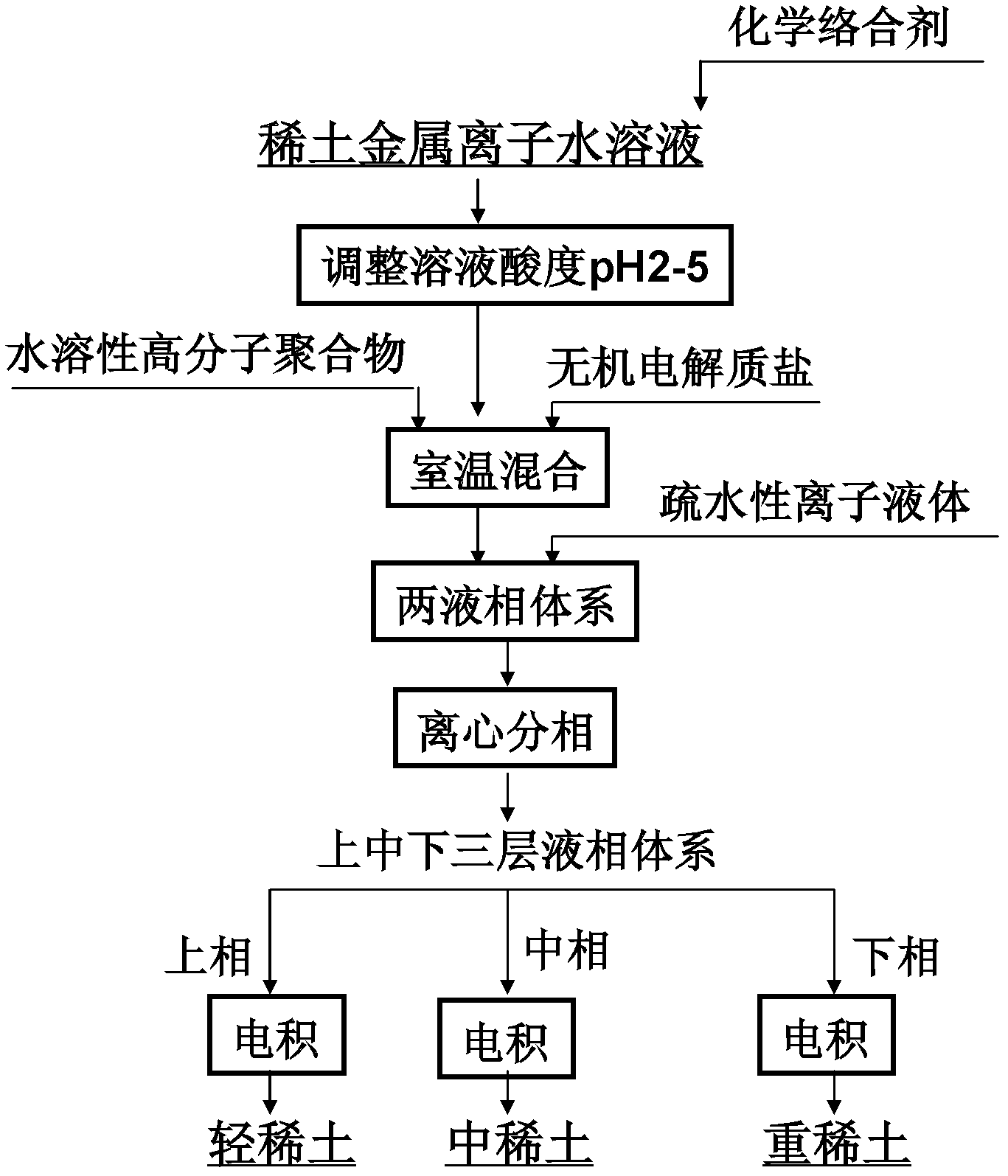
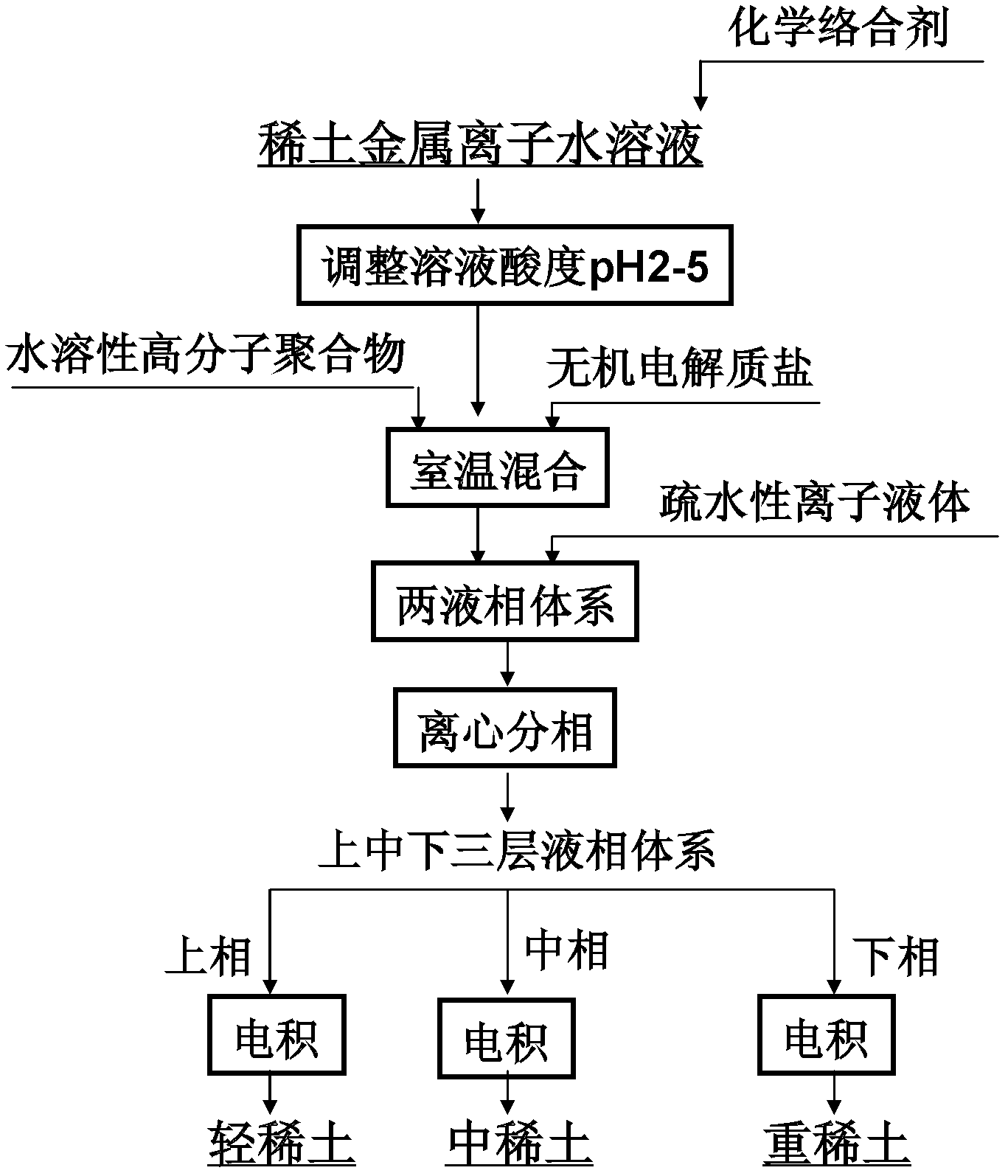
[0033] Dissolve the oxides of lanthanum, europium, and ytterbium with 5 mL of 0.1 mol / L aqueous hydrochloric acid solution to form a mixed solution containing lanthanum, europium, and ytterbium ions (the initial concentrations of the three rare earth metal ions are all 100 mg / L). EDTA is added, and the ratio of the added molar amount to the sum of the molar amounts of the three rare earth ions in the initial aqueous solution is 1:1. Adjust the pH of the mixed solution to 5, and stir thoroughly at room temperature. Then add EOPO 2500 and sodium sulfate, EOPO accounts for 20% by weight in the mixed solution, and sodium sulfate accounts for 30% by weight. After fully shaking and mixing at room temperature, centrifugation is carried out to obtain a two-liquid phase system in which upper and lower layers coexist. Then, 5 mL of ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) was added to the above-mentioned two-liquid phase system, and the mixture was ce...
Embodiment 2
[0035] Dissolve the oxides of lanthanum, europium, and lutetium with 5 mL of 0.1 mol / L nitric acid aqueous solution to prepare a mixed solution containing lanthanum, europium, and lutetium ions (the initial concentration of the three rare earth metal ions is 100 mg / L). EGTA is added, and the ratio of the added molar amount to the sum of the molar amounts of the three rare earth ions in the initial aqueous solution is 1.5:1. Adjust the pH of the mixed solution to 2, and stir thoroughly at room temperature. Then add PEG 2000 and potassium nitrate, PEG2000 accounts for 10% by weight in the mixed solution, and potassium nitrate accounts for 20% by weight. After fully shaking and mixing at room temperature, centrifugation is carried out to obtain a two-liquid phase system in which upper and lower layers coexist. Then, 10 mL of ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) was added to the above-mentioned two-liquid phase system, and the mixture was ce...
Embodiment 3
[0037] Dissolve the oxides of lanthanum, europium, and lutetium with 5 mL of 0.1 mol / L aqueous hydrochloric acid solution to form a mixed solution containing lanthanum, europium, and lutetium ions (the initial concentrations of the three rare earth metal ions are all 100 mg / L). Add PAA, the ratio of the added molar amount to the sum of the molar amounts of the three rare earth ions in the initial aqueous solution is 2:1. Adjust the pH of the mixed solution to 5, and stir thoroughly at room temperature. Then add EOPO 2500 and sodium chloride, EOPO2500 accounts for 30% by weight in the mixed solution, and sodium chloride accounts for 30% by weight. After fully shaking and mixing at room temperature, centrifugation is carried out to obtain a two-liquid phase system in which upper and lower layers coexist. Then, add 15mL ionic liquid [C to the above-mentioned two-liquid phase system 9 h 14 N][N(CF 3 SO 2 ) 2 ], fully oscillating and mixing at room temperature, and centrifugi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com