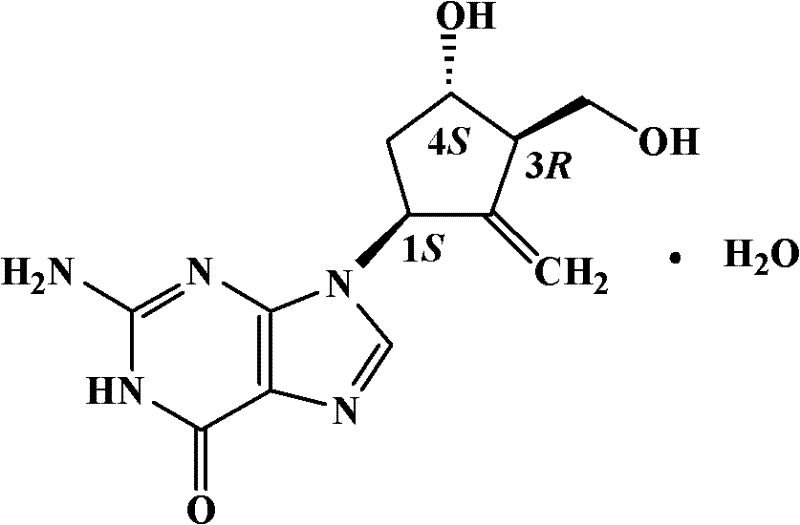
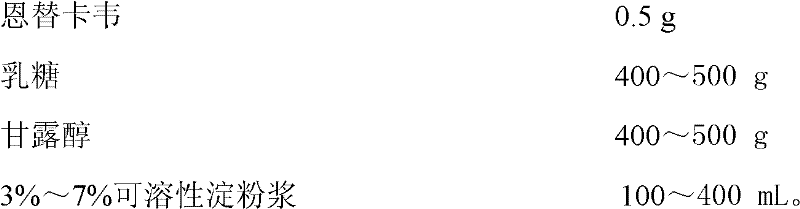Entecavir granule formulation and preparation method thereof
A technology of entecavir and granules, which is applied in the field of entecavir soluble granules and its preparation, can solve the problems of affecting the bioavailability of active components and poor solubility of granules, and achieve solid and non-brittle granules, fast onset of action, good taste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation Components of Entecavir Granules:
[0051]
[0052] The preparation method of entecavir granules:
[0053] (1) Pulverize entecavir, lactose and mannitol respectively, pass entecavir through a 120-mesh sieve, and lactose and mannitol pass through a 100-mesh sieve;
[0054] (2) prepare 5% soluble starch slurry;
[0055] (3) First mix mannitol and lactose evenly, then weigh the above excipient mixture equivalent to entecavir, grind it with entecavir for 15 minutes, then add the mixture of mannitol and lactose in increments, mix evenly, and finally use 5% wet granules made of soluble starch slurry;
[0056] (4) The wet granules obtained in step (3) were dried at 55° C., and then sized to obtain the entecavir granules.
Embodiment 2
[0058] Preparation components of entecavir granules (specification: 0.5mg / bag):
[0059]
[0060] The preparation method of entecavir granules:
[0061] (1) Pulverize entecavir, sucrose and soluble starch respectively, pass entecavir through a 120 mesh sieve, and sucrose and soluble starch pass through a 100 mesh sieve respectively;
[0062] (2) prepare 3% hypromellose aqueous solution;
[0063] (3) firstly mix sucrose and soluble starch evenly, then, mix entecavir with the above-mentioned mixture uniformly in increments, and finally use 3% hypromellose aqueous solution to make wet granules;
[0064] (4) The wet granules obtained in step (3) were dried at 55° C., and then sized to obtain the entecavir granules.
Embodiment 3
[0066] Preparation components of Entecavir granules (specification: 1mg / bag):
[0067]
[0068] The preparation method of entecavir granules:
[0069] (1) Pulverize entecavir, xylitol and lactose respectively, pass entecavir through a 120 mesh sieve, and xylitol and lactose pass through a 100 mesh sieve respectively;
[0070] (2) prepare 5% hypromellose 50% ethanol solution;
[0071] (3) Mix xylitol and lactose uniformly first, then mix entecavir and the above-mentioned mixture uniformly in equal increments, and finally use 5% hypromellose 50% ethanol solution to prepare wet granules;
[0072] (4) The wet granules obtained in step (3) were dried at 55° C., and then sized to obtain the entecavir granules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com