Preparation method of solid-solid phase-change material immobilized by chemical cross-linking method
A chemical cross-linking method and phase change material technology, applied in the field of phase change energy storage materials, can solve the problems of rare application, unacceptable, high cost, etc., and achieve the effect of simple process, low production cost and good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve 3g of PEGA, 0.024g of N,N-methylenebisacrylamide, and 0.03g of ammonium persulfate in 21ml of deionized water, react in a water bath at 75°C for 3h, and dry in a vacuum oven at 35°C for 24h to obtain crosslinking A lesser degree of gel.
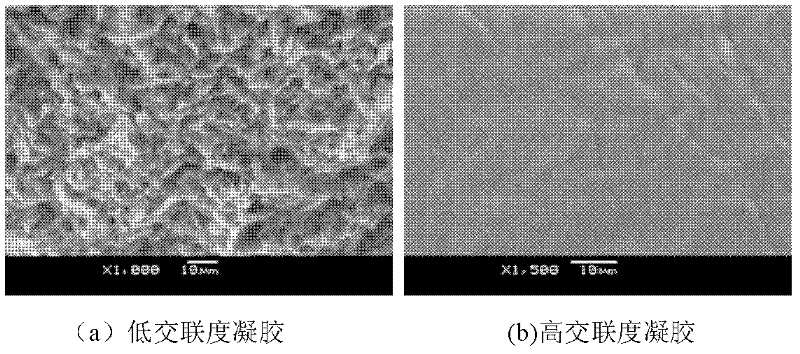
[0029] Carry out SEM analysis to the section of above-mentioned phase change material [see figure 1 (a)], the phase change material prepared in this example has an obvious loose network structure and no phase separation, indicating that the product achieves chemical permanent immobilization of the phase change material in the form of a network.
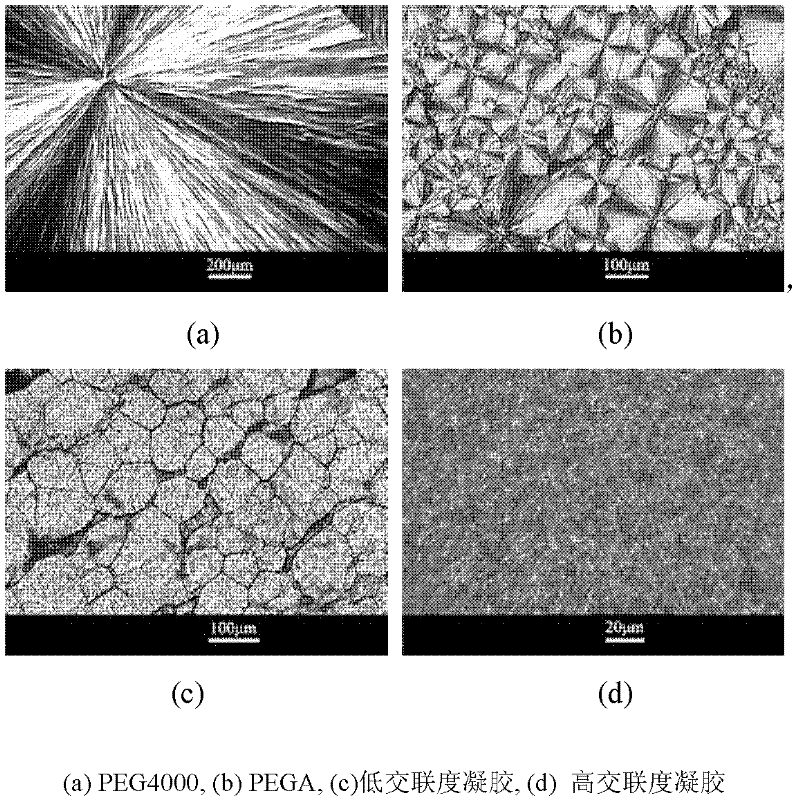
[0030] Through the observation of the crystal morphology of phase change materials, it is found that [see figure 2 (c)], due to the existence of a small number of cross-linking points, the length of the free-moving segment of the macromolecular chain is reduced when the macromolecular chain is crystallized, and the regular arrangement position is limited. It can be seen from the fig...
Embodiment 2
[0035] Dissolve 3g of PEGA, 0.03g of N,N-methylenebisacrylamide, and 0.015g of ammonium persulfate in 21ml of deionized water, react in a water bath at 75°C for 4h, and dry in a vacuum oven at 35°C for 24h to obtain crosslinking A lesser degree of gel.
Embodiment 3
[0037] Dissolve 4g of PEGA, 1.4g of N, N-methylenebisacrylamide, and 0.06g of ammonium persulfate in 24ml of deionized water, react in a water bath at 75°C for 3h, and dry in a vacuum oven at 35°C for 24h to obtain a crosslinked Higher degree of gel.
PUM
| Property | Measurement | Unit |
|---|---|---|
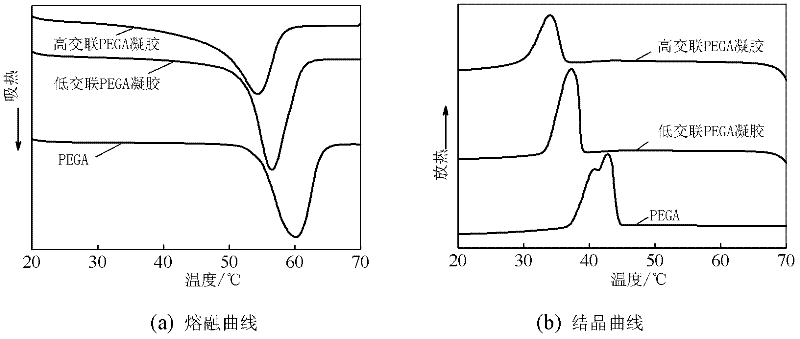
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com