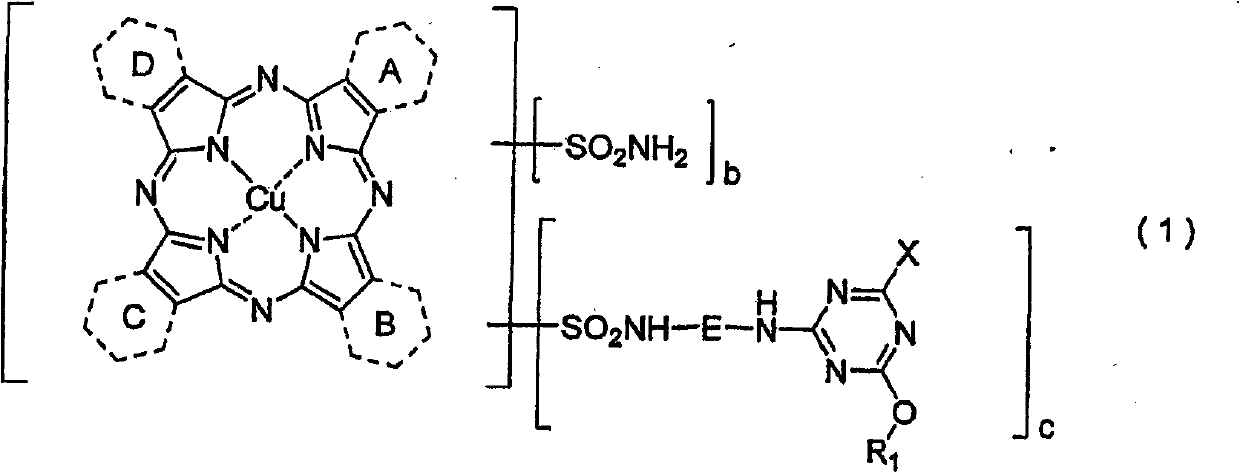
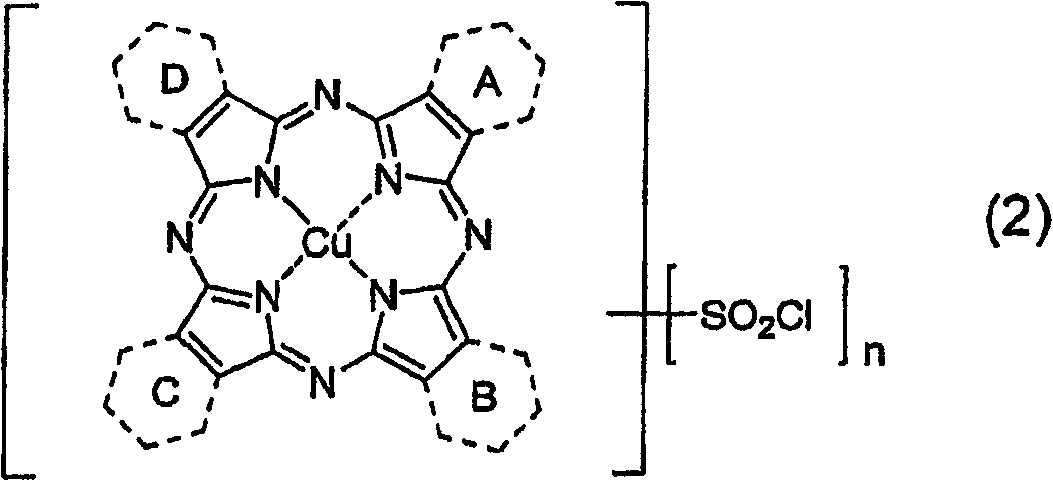
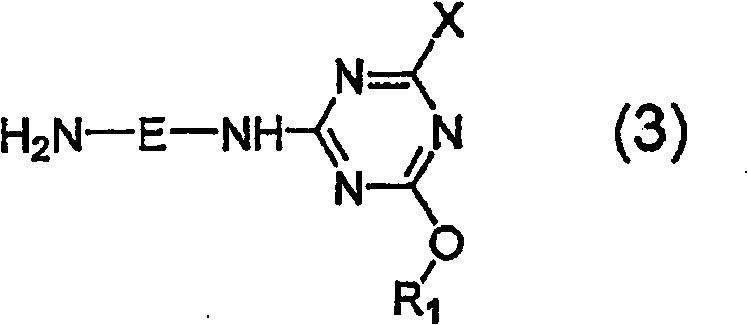
Porphyrazine dye, ink composition containing same, and colored body
A technology of porphyrazine and pigments, which is applied in the directions of inks, azo dyes, organic dyes, etc., can solve the problem that the color tone, printing density light resistance, ozone gas resistance and moisture resistance cyan pigments have not been satisfied, and the cyan pigments have not been fully satisfied. Market requirements and other issues, to achieve a good balance between fastness and quality, not easy to bronze phenomenon, and reduce printing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0318] (step 1)
[0319] Synthesis of a compound represented by the following formula (4) in which 1.37 of the rings A to D in the following formula (4) are pyrazine rings fused at the 2nd and 3rd positions, and the remaining 2.63 are benzene rings
[0320]
[0321] 29.16 parts of phthalic anhydride, 17.23 parts of quinolinic acid, 108 parts of urea, 10.1 parts of copper (II) chloride and 1.5 parts of ammonium molybdate were added to 375 parts of sulfolane. The liquid temperature was raised to 200° C., and the reaction liquid was maintained at this temperature for 5 hours. After completion of the reaction, the reaction liquid was cooled to 65° C., 50 parts of DMF (N,N-dimethylformamide) was added thereto, and the precipitated solid was separated by filtration. The resulting solid was washed with 50 parts of DMF to obtain 75.1 parts of a wet cake. The resulting wet cake was added to 450 parts of DMF, the temperature of the liquid was raised to 110°C, and maintained at this...
Embodiment 2
[0340] (step 1)
[0341] Synthesis of a compound represented by the aforementioned formula (4) in which 1.00 of the rings A to D in the aforementioned formula (4) are pyridine rings fused at the second and third positions and the remaining 3.00 are benzene rings
[0342] 33.32 parts of phthalic anhydride, 10.08 parts of quinolinic acid, 108 parts of urea, 10.1 parts of copper (II) chloride and 1.5 parts of ammonium molybdate were added to 375 parts of sulfolane. The liquid temperature was raised to 200° C., and the reaction liquid was maintained at this temperature for 5 hours. After completion of the reaction, the reaction liquid was cooled to 65° C., 50 parts of DMF (N,N-dimethylformamide) was added thereto, and the precipitated solid was separated by filtration. The obtained solid was washed with 50 parts of DMF to obtain 75.1 parts of a wet cake. The obtained wet cake was added to 450 parts of DMF, the temperature of the liquid was raised to 110° C., and this temperature...
Embodiment 3
[0352] (step 1)
[0353] Synthesis of a compound represented by the aforementioned formula (4) in which 0.75 of the rings A to D in the aforementioned formula (4) are pyridine rings fused at the 2nd and 3rd positions and the remaining 3.25 are benzene rings
[0354] 36.1 parts of phthalic anhydride, 9.4 parts of quinolinic acid, 108 parts of urea, 10.1 parts of copper (II) chloride, and 1.5 parts of ammonium molybdate were added to 375 parts of sulfolane. The liquid temperature was raised to 200° C., and the reaction liquid was maintained at this temperature for 5 hours. After completion of the reaction, the reaction liquid was cooled to 65° C., 50 parts of DMF (N,N-dimethylformamide) was added thereto, and the precipitated solid was separated by filtration. The obtained solid was washed with 50 parts of DMF to obtain 75.1 parts of a wet cake. The obtained wet cake was added to 450 parts of DMF, the liquid temperature was raised to 110° C., and this temperature was maintaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com