Tumor-targeting double-drug carrying and delivery system and preparation method thereof
A tumor-targeting, dual-loading technology, which is applied in the field of biotechnology and pharmaceutical preparations, can solve the problems affecting tumor targeting efficiency and high concentration, and achieve the effects of high targeting and treatment efficiency, simple preparation, and avoiding interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
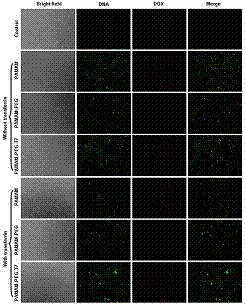
Examples
Embodiment 1
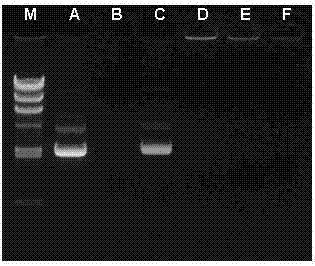
[0038] Take 3 mg of polyamide-amine (PAMAM, 77.35 mg / ml methanol solution) and blow dry in a vial, add 0.73 mg of polyethylene glycol (MAL-PEG3500-NHS, 1 mg / ml 0.035 M pH 8.0 phosphate solution) , The molar ratio of the two is 1:2, and the reaction is stirred at room temperature for 2 h. The NHS group reacts specifically with the amino group on the surface of PAMAM to produce polyamide-amine-polyethylene glycol (PAMAM-PEG). 2 ) Complex solution, MWCO 10000 ultrafiltration centrifuge tube at 12000 rpm for 30 min to remove unreacted polyethylene glycol (PEG3500), and hydrogen nuclear magnetic resonance spectrum to characterize the synthesis of the carrier.
Embodiment 2
[0040] Take 3 mg of polyamide-amine (PAMAM, 77.35 mg / ml methanol solution) and blow dry in a vial, add 0.73 mg of polyethylene glycol (MAL-PEG3500-NHS, 1 mg / ml 0.035 M pH 8.0 phosphate solution) , The molar ratio of the two is 1:2, and the reaction is stirred at room temperature for 2 h. The NHS group reacts specifically with the amino group on the surface of PAMAM to produce polyamide-amine-polyethylene glycol (PAMAM-PEG). 2 ) Complex solution, MWCO 10000 ultrafiltration centrifuge tube at 12000 rpm for 30 min to remove unreacted polyethylene glycol (PEG3500), pH 7.0 phosphate buffer solution for reconstitution, add 0.10 mg of peptide (T7, 2 mg / ml pH 7.0 phosphate solution), the molar ratio with PAMAM is 1:1, and the reaction is stirred at room temperature for 24 hours. The MAL group reacts specifically with the sulfhydryl group on the cysteine residue of the polypeptide (T7) to produce polyamide— Amine-polyethylene glycol-polypeptide (PAMAM-PEG-T7) complex, MWCO 10000 ultra...
Embodiment 3
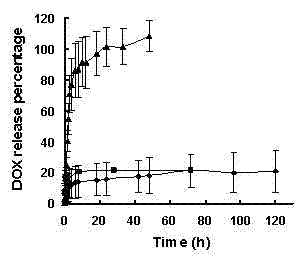
[0042] Take an appropriate amount of doxorubicin methanol solution in a vial and blow dry, add a pH 7.4 phosphate buffer to make a 3.48 μg / ml doxorubicin solution, take 0.5 ml in a centrifuge tube, and use LS-55 fluorescence The spectrometer was excited at 480 nm, and the fluorescence emission spectrum of adriamycin was detected in the range of 520-680 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com