A glutathione-responsive targeting polymer micelle and its preparation method and application
A technology of glutathione and polymer, applied in the field of biomedical materials, can solve the problem that double drug-loaded polymer micelles have not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
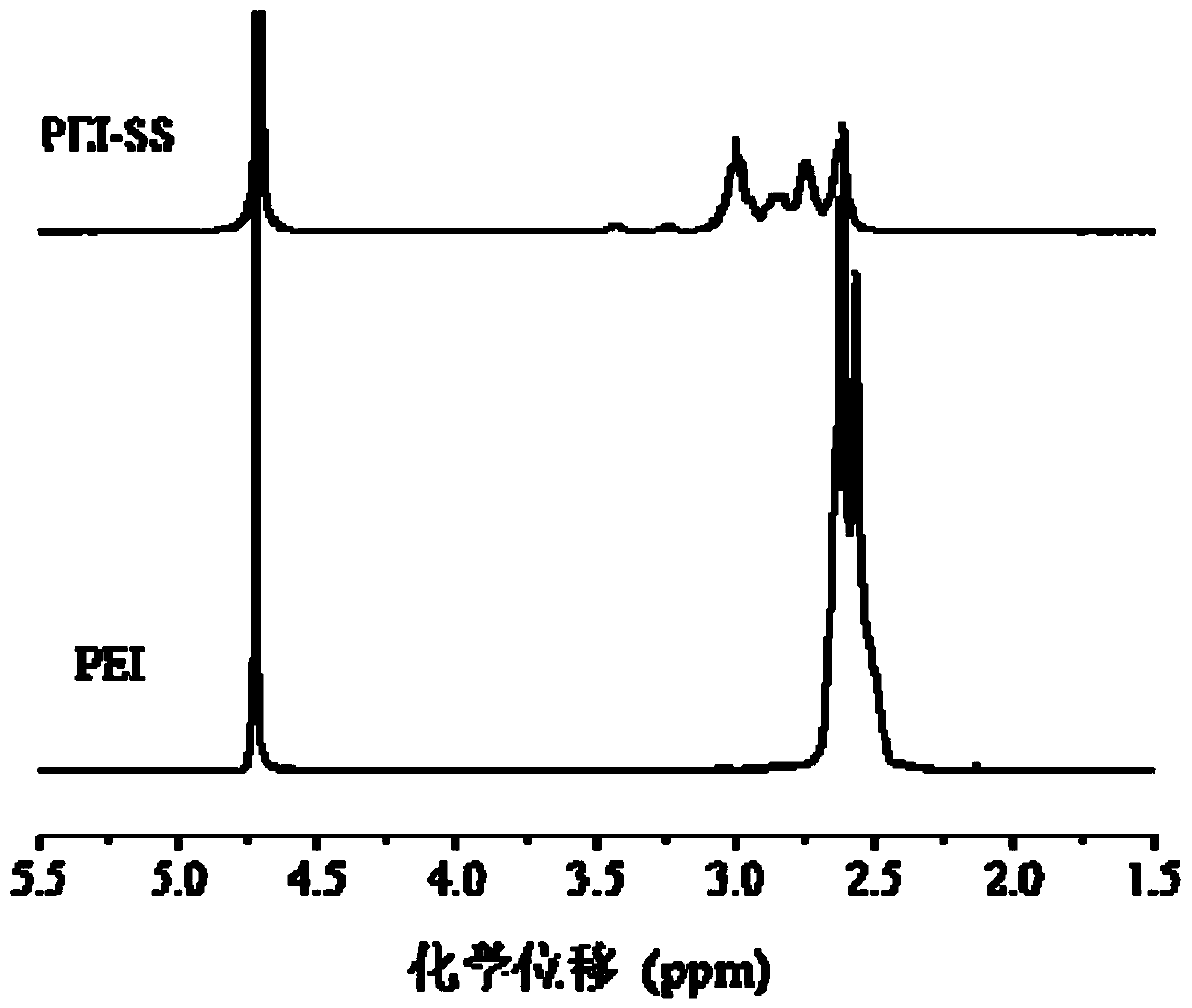
[0089] Example 1: Synthesis of disulfide bond cross-linked polyethyleneimine (PEI-SS)
[0090] Dissolve polyethyleneimine (PEI) in methanol aqueous solution, and slowly drop the methanol solution of N,N'-bis(acryloyl)cystamine (CBA) into the PEI solution under nitrogen and protected from light. The addition time is 30min, the water bath is heated and stirred at 45°C for 4h, and the speed is 400rpm; then a small amount of polyethyleneimine (PEI) solution is added again to make N,N'-bis(acryloyl)cystamine (CBA) fully react. After 2h, add deionized water and return to room temperature to terminate the reaction. Adjust the pH value to 4.0, dialyze for three days, freeze-dry for 24 hours to obtain disulfide bond cross-linked polyethyleneimine (PEI-SS).
[0091] The molar ratio of the polyethyleneimine (PEI) and N,N'-bis(acryloyl)cystamine (CBA) is 6:1; the concentration of the polyethyleneimine (PEI) in the reaction system It is 0.04 mol / L, and the volume of deionized water used to te...
Embodiment 2
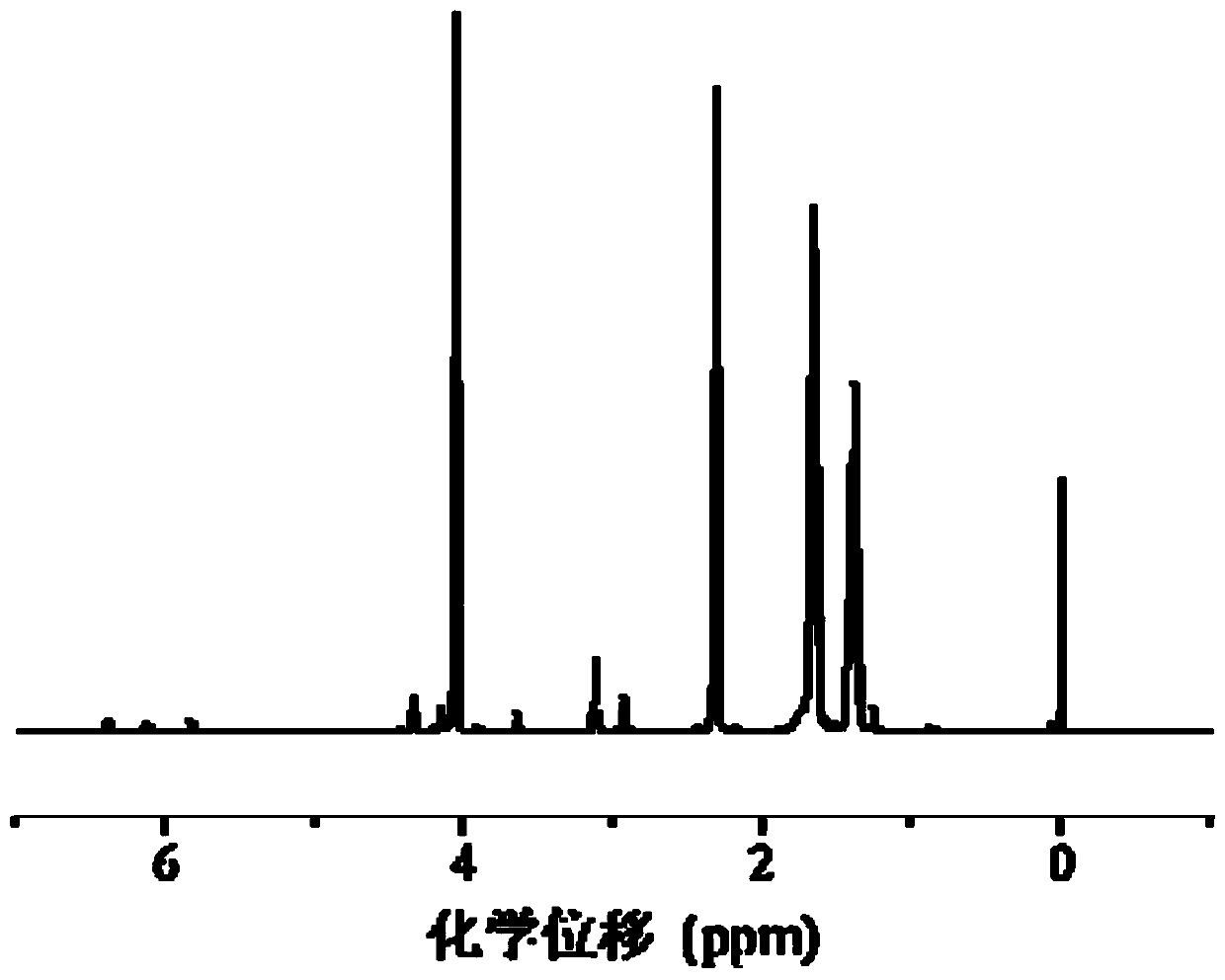
[0092] Example 2: Synthesis of disulfide bond cross-linked polyethyleneimine (PEI-SS)
[0093] Dissolve polyethyleneimine (PEI) in methanol aqueous solution, and slowly drop the methanol solution of N,N'-bis(acryloyl)cystamine (CBA) into the PEI solution under nitrogen and protected from light. The addition time is 30min, the water bath is heated and stirred at 45°C for 4h, and the speed is 400rpm; then a small amount of polyethyleneimine (PEI) solution is added again to make N,N'-bis(acryloyl)cystamine (CBA) fully react. After 2h, add deionized water and return to room temperature to terminate the reaction. Adjust the pH value to 4.0, dialyze for three days, freeze-dry for 24 hours to obtain disulfide bond cross-linked polyethyleneimine (PEI-SS).
[0094] The molar ratio of the polyethyleneimine (PEI) and N,N'-bis(acryloyl)cystamine (CBA) is 4:1; the concentration of the polyethyleneimine (PEI) in the reaction system It is 0.02 mol / L, and the volume of deionized water used to te...
Embodiment 3
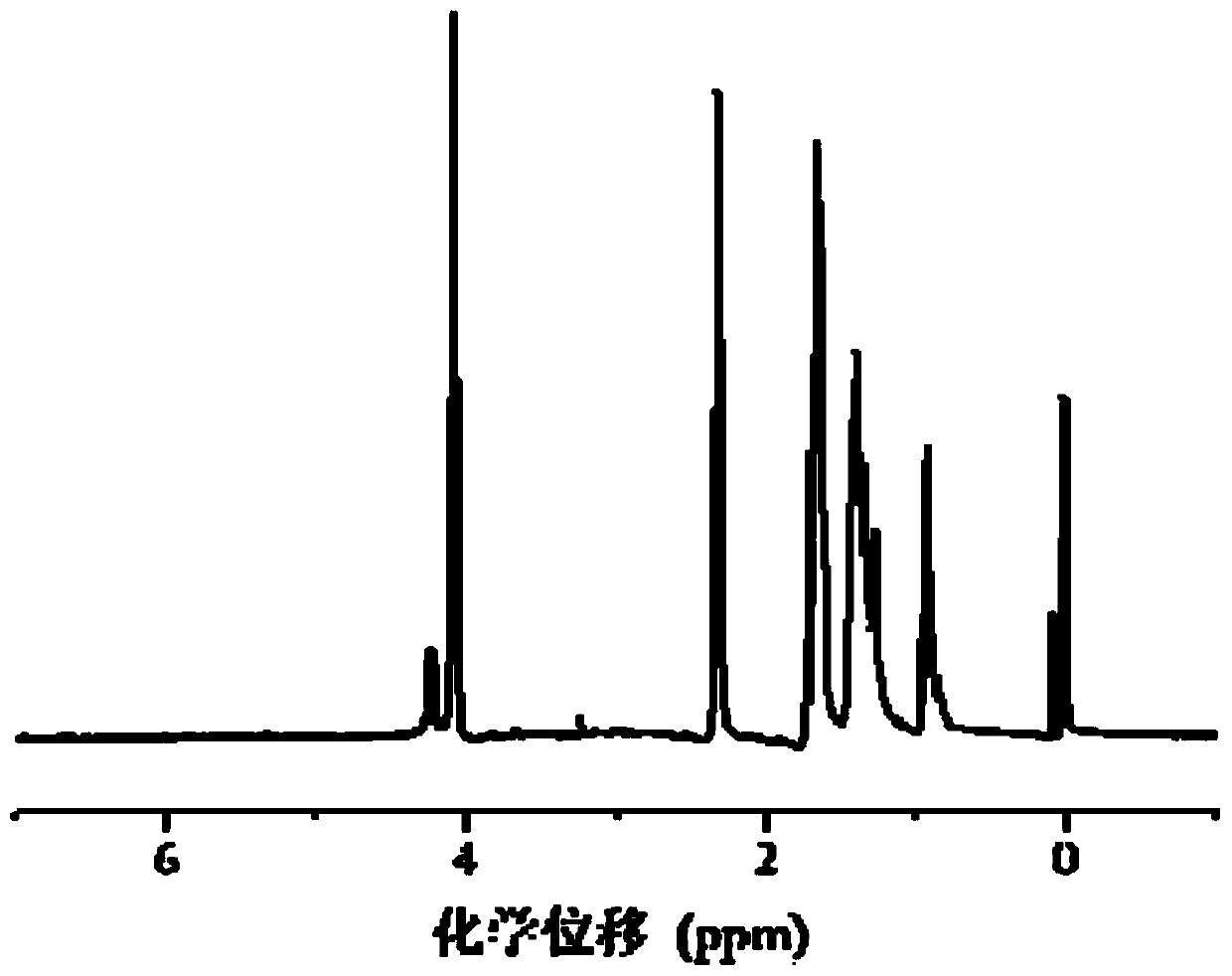
[0095] Example 3: Synthesis of disulfide bond cross-linked polyethyleneimine (PEI-SS)
[0096] Dissolve polyethyleneimine (PEI) in methanol aqueous solution, and slowly drop the methanol solution of N,N'-bis(acryloyl)cystamine (CBA) into the PEI solution under nitrogen and protected from light. The addition time is 30min, the water bath is heated and stirred at 45°C for 4h, and the speed is 400rpm; then a small amount of polyethyleneimine (PEI) solution is added again to make N,N'-bis(acryloyl)cystamine (CBA) fully react. After 2h, add deionized water and return to room temperature to terminate the reaction. Adjust the pH value to 4.0, dialyze for three days, freeze-dry for 24 hours to obtain disulfide bond cross-linked polyethyleneimine (PEI-SS).
[0097] The molar ratio of the polyethyleneimine (PEI) and N,N'-bis(acryloyl)cystamine (CBA) is 8:1; the concentration of the polyethyleneimine (PEI) in the reaction system It is 0.06mol / L, and the volume of deionized water used to ter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com