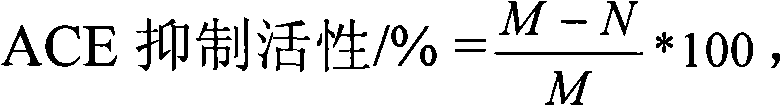Tripeptides with angiotensin converting enzyme inhibition activity and their use and composition
An angiotensin and activity-inhibiting technology, which is applied in the field of functional foods and pharmaceutical compositions, can solve problems such as poor patient compliance, and achieve the effect of inhibiting ACE activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of the tripeptide provided by the present invention includes but not limited to: separating the polypeptide from the hydrolyzate by hydrolyzing the milk protein; or adopting a chemical synthesis method. The chemical synthesis method has unique advantages in the synthesis of small molecular polypeptides, and can quickly synthesize a high-purity single sequence in a short period of time, so the chemical synthesis method is preferred.
[0018] The chemical synthesis method can be tBoc solid-phase synthesis method or Fmoc solid-phase synthesis method, preferably Fmoc solid-phase synthesis method. The present invention can utilize the principle, steps and operating conditions of the Fmoc solid-phase synthesis method described in detail in Merrifield B. Solid phase synthesis [J]. Science, 1986, 232 (4748): 341-347.
[0019] The present invention also provides compositions containing at least one tripeptide described in the present invention as an active...
Embodiment 1
[0029] This example is used to illustrate the preparation process of the tripeptide of the present invention.
[0030] (1) Fmoc solid-phase synthesis process
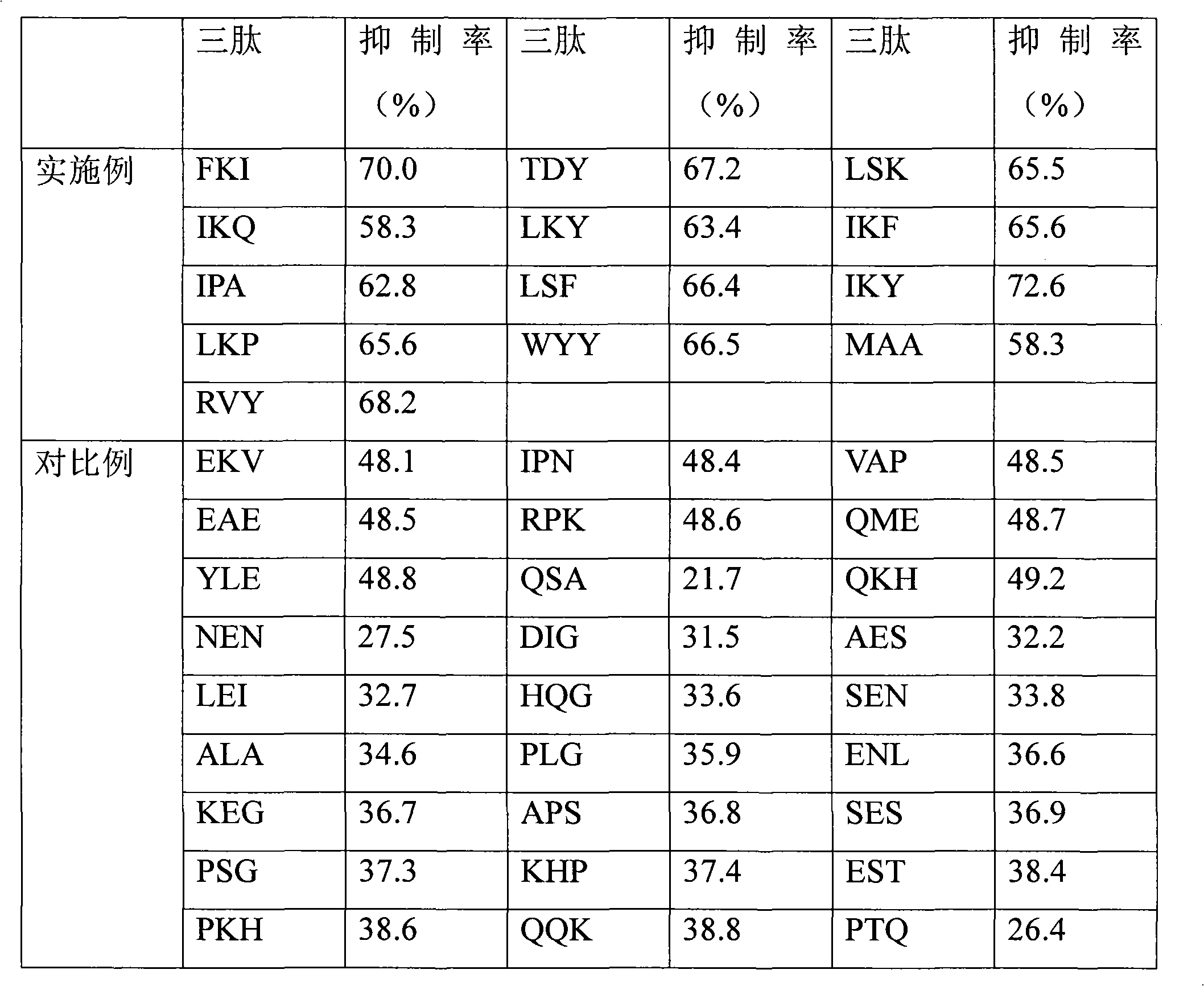
[0031] Referring to the principle, steps and operating conditions of the Fmoc solid-phase synthesis method described in detail in Merrifield B. Solid phase synthesis [J]. Science, 1986, 232 (4748): 341-347, synthesize TDY, FKI, LSK, and LKY in sequence , IKF, IPA, IKY, LSF, LKP, WYY, RVY, IKQ or MMA.
[0032] (2) Purification of tripeptide
[0033] The tripeptide synthesized in step (1) was purified by semi-preparative reversed phase high performance liquid chromatography (RP-HPLC) under the following conditions. A Shimadzu (LC-10A) liquid chromatograph was used with BECKMAN C18 preparative column, mobile phase A: 0.1% trifluoroacetic acid (TFA) / ultrapure water; mobile phase B: 0.1% TFA / acetonitrile. Gradient program: 5%-50% B for 30min, flow rate 5mL / min, sample volume 3mL, use 230nm wavelength to detect and collect...
Embodiment 2
[0040] This example is used to determine the ACE inhibitory activity of the tripeptide prepared in Example 1.
[0041]Referring to the assay method of ACE inhibitory activity established by Wu et al. in 2002 (Wu J P, Aluko R E, Muir A D. Improved method for direct high-performance liquid chromatography assay of angiotensin-converting enzyme-catalyzed reactions[J].Journal of Chromatography A, 2002, 950: 125-130.). Take 130 μL of 5 mmol / L HHL solution, add 20 μL of 20 mmol / L tripeptide, mix well, and keep warm in a constant temperature water bath at 37°C for 10 min. Then 10 μL of ACE enzyme solution was added, reacted in a constant temperature water bath at 37° C. for 30 min, and then 150 μL of 1 mol / L HCl was added to terminate the reaction to obtain a reaction solution. The results were analyzed by HPLC system. At the same time, 20 μL of 0.1mol / L phosphate buffer was used instead of tripeptide as a control group. The formula for calculating ACE inhibitory activity is as fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com