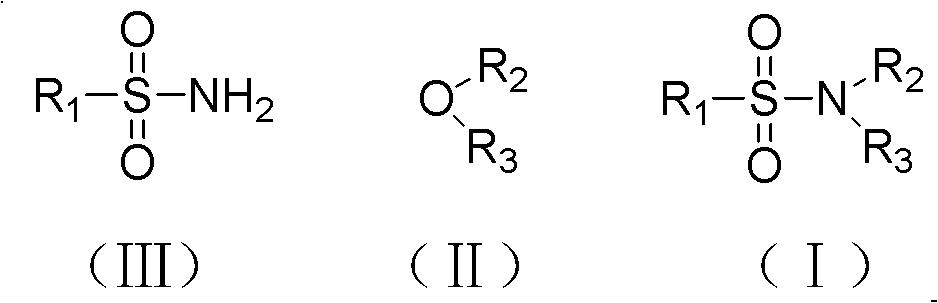
Preparation method of N,N-disubstituted sulfonamide compound
A technology of sulfonamide and ether compounds, applied in the field of N, can solve the problems of limited application range, harsh conditions, expensive raw materials, etc., and achieve the effects of wide industrial application prospects, mild reaction conditions, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
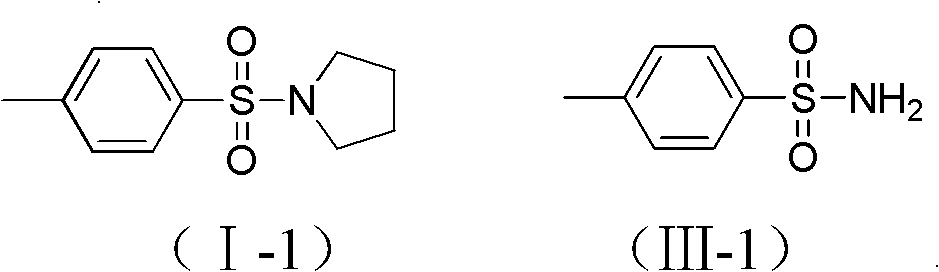
[0018] Example 1: N-p-toluenesulfonyl tetrahydropyrrole (I-1)
[0019] Mix 171.3mg (1mmol) of p-toluenesulfonamide (III-1) and 0.32ml (4mmol) of tetrahydrofuran, in 2ml of toluene, under the action of 20μl (0.2mmol) of trifluoromethanesulfonic acid, stir the reaction at 170℃18 After the reaction was completed, the reaction solution was distilled to remove the solvent, and then column chromatography (eluent: petroleum ether: ethyl acetate = 5:1), TLC followed to collect the eluent with Rf value of 0.3~0.35 The collected eluent was distilled under reduced pressure and dried to obtain 220.8 mg of target compound (I-1), with a yield of 98.1%, a white solid.
[0020] 1 H NMR(500MHz, CDCl 3 ): δ1.76-1.73(m, 4H), 2.43(s, 3H), 3.14-3.21(m, 4H), 7.32(d, 2H, J=8.5Hz), 7.71(d, 2H, J=8.5 Hz).
[0021]
Embodiment 2
[0023] The amount of tetrahydrofuran was increased to 2ml (25mmol), other operations were the same as in Example 1, and the yield was 96.6%.
Embodiment 3
[0025] The reaction temperature was changed to 30°C, the reaction time was 48 hours, the other operations were the same as in Example 1, and the yield was 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com